CPHI Europe: Gummies as a drug delivery system could improve compliance
Pharmaceutical Technology
OCTOBER 10, 2024
At CPHI Europe, an expert discussed the promise of gummies as a drug delivery system and their unique manufacturing challenges.
This site uses cookies to improve your experience. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country, we will assume you are from the United States. Select your Cookie Settings or view our Privacy Policy and Terms of Use.
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.
Used for the proper function of the website
Used for monitoring website traffic and interactions
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.

Pharmaceutical Technology
OCTOBER 10, 2024
At CPHI Europe, an expert discussed the promise of gummies as a drug delivery system and their unique manufacturing challenges.
XTalks
JANUARY 29, 2025
Highlights include expansion of biotech operations in Philadelphia, acquisition of a new pharma-device facility near Dublin, Ireland, and a new Center of Excellence for advanced drug delivery and drug-device combination product assembly in Rockford, Illinois.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
Roots Analysis
FEBRUARY 26, 2023
Over the last two decades, the pharmaceutical industry has observed a paradigm shift from conventional drug delivery strategies to the long-acting drug delivery (LADD) of products to treat several disease indications, such as ophthalmic disorders, oncological disorders, neurological disorders and infectious diseases.

pharmaphorum
AUGUST 5, 2020
Workshop will be on ‘Ophthalmic Drug Delivery Development – Trials and Tribulations’. Ophthalmic Drug Delivery is compelling for many reasons, including the major issue of patient compliance and tolerability of therapies. Hosted by Brian Levy, CEO, Ocunexus Therapeutics.

Outsourcing Pharma
AUGUST 14, 2024
In an increasingly complex regulatory landscape, pharmaceutical companies face significant challenges in maintaining transparency and accountability across their global supply chains.

Pharmaceutical Technology
FEBRUARY 16, 2023
The ingredient size reduction might help expand the universe of existing and future drugs that may benefit from drug delivery using the Optejet dispenser.

Roots Analysis
SEPTEMBER 18, 2023
Therefore, medical technology is rapidly evolving, enabling more advanced and patient-friendly methods of drug delivery. In the pursuit of creating patient-centric healthcare solutions, the medical industry has witnessed a groundbreaking advancement in drug delivery with the emergence of large volume wearable injectors.

pharmaphorum
OCTOBER 20, 2015
s Chris Evans and HealthPrize Technologies' Katrina Firlik to learn about how their organizations are collaborating to provide an end-to-end connected health solution that tracks, educates, motivates and engages patients to increase adherence and medical literacy and rewards them for compliance with their prescribed regimen.

XTalks
SEPTEMBER 23, 2020
And in turn, these feelings and experiences of anxiousness can affect medication compliance and adherence. This highlights the importance of optimal drug delivery, including the injection experience itself.

pharmaphorum
MARCH 14, 2022
This morning, Sandoz announced it has acquired medical and drug delivery device specialist Coalesce Product Development, bolstering its capabilities for the development of respiratory medicines and ‘complex’ generics.

Camargo
OCTOBER 14, 2020
Approval of the Month: FDA Approves First Closed-Loop Monitoring and Drug Delivery Device. The FDA has announced its approval of a bluetooth-controlled insulin delivery and monitoring system for use in pediatric patients ages 2 through 6 with Type 1 diabetes. Senior Director of Regulatory Compliance and Submissions.

pharmaphorum
OCTOBER 12, 2022
Sustainability for Injectable Delivery Devices. Connected Drug Delivery Devices. Els Ducheyne, Senior Manager, Materials Compliance, Johnson and Johnson. Fatima Bennai-Sanfourche, Senior Director of QA & RA Compliance for Medical Device, Combination Products and eHealth, Bayer. Primary Packaging Development.

pharmaphorum
NOVEMBER 17, 2021
The Future of Drug Delivery and Combination Product Device Design. The pre-filled syringes industry is growing at an exponential rate with innovations in parenteral delivery device development to aid self-administration and deliver biologics, high concentration, and large-volume drug products.
pharmaphorum
JANUARY 12, 2021
They also offer the potential for self-administration at home rather than in a health setting, making compliance with booster dosage potentially higher.”. “Non-injectables remove the need for health professional-led immunisation programmes, making widespread vaccine roll-outs quicker and easier and more affordable,” Channon told pharmaphorum.

Roots Analysis
FEBRUARY 22, 2022
Autoinjector As Emerging Drug Delivery Device. The field is presently witnessing several innovations, such as LED / LCD-based visualization, Bluetooth connectivity, dosage recording, safety lock, visual / audible drug delivery confirmation notifications, and automatic drug reconstitution.

XTalks
NOVEMBER 30, 2023
For instance, drug delivery devices may meet all technical requirements regarding dosage and delivery methods but fail in practical use by patients. These failures usually result from a development process focusing only on technical aspects and healthcare provider perspectives, neglecting patient input.

The Pharma Data
OCTOBER 27, 2020
3a will continue to advance its industrial design, product usability, labelling compliance, regulatory approval, and production planning work in parallel to its validation studies. .” Final validation studies are planned to be carried out for both RNA test systems in Germany in Q4 2020. About XPhyto Therapeutics Corp.

Roots Analysis
MAY 24, 2024
In the dynamic landscape of the healthcare sector, the evolution of drug delivery systems plays a significant role in transforming the management of chronic diseases. These devices reduce injection anxiety, and are useful for patients who prefer a hands-free drug delivery experience.

The Pharma Data
DECEMBER 20, 2020
With this acquisition we are expanding our product pipeline to include psychedelic therapeutics, incorporating elements of our IP around drug delivery technology in which we already have prototypes developed, which we believe will propel us towards clinical studies relatively quickly. Ahmad Doroudian, CEO of BetterLife.

Roots Analysis
JANUARY 17, 2024
They offer several advantages over traditional drug delivery systems ( such as vials and syringes ), including reduced chances of dosing errors, increased patient compliance and decreased risk of microbial contamination. Prefilled syringes are syringes that come pre-filled with a specific dose of medication.

XTalks
AUGUST 30, 2022
Public health and regulatory compliance entities depend on bureaucratic leadership to run effectively. Big pharmaceutical companies require a system of hierarchy to accurately adhere to the legal and regulatory requirements for drug delivery, drug approval, supply chain and manufacturing processes.
XTalks
JUNE 11, 2024
Drug-eluting stents (DES): DES reduce the incidence of restenosis and are coated with drugs that inhibit scar tissue growth. DCBs: DCBs, such as the Agent DCB, combine mechanical widening of the artery with drug delivery to the vessel wall to prevent cell proliferation.

XTalks
JULY 5, 2022
A corporate compliance training program is a part of many onboarding programs to ensure employees are up to date with the latest rules and regulations they must adhere to while working for the company. Novartis trains and guides associates with a structured program consisting of ethics, risk and compliance.

The Pharma Data
DECEMBER 28, 2020
is an emerging biotechnology company engaged in the development and commercialization of therapeutic pharmaceuticals as well as drug delivery platform technologies. About BetterLife Pharma Inc. BetterLife Pharma Inc.

The Pharma Data
OCTOBER 14, 2020
15, 2020 /PRNewswire/ — Seattle Gummy Company’s breakthrough gummy drug program, which delivers Active Pharmaceutical Ingredients (APIs) in its innovative gummy matrix, recently won its first Investigational New Drug Application (IND) approval from FDA on the company’s allergy gummy medication, designated as IND 140312.

The Pharma Data
DECEMBER 17, 2020
Administration of Buvidal is restricted to healthcare professionals, increasing treatment compliance, and minimizing risks of diversion, misuse and pediatric exposure. Buvidal received market authorizations in EU and Australia in November 2018. About Camurus.

FDA Law Blog
JANUARY 5, 2023
Claud — As we turn into the New Year, we offer a few items of interest in digital and telehealth regulation, enforcement, and compliance that may provide some helpful guideposts for stakeholders. By John W.M. Remarks at the November AFDO/RAPS Combination Products Summit highlight the complexities of connected combination products.
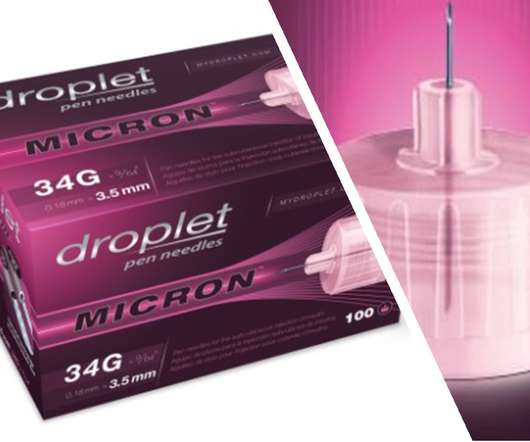
XTalks
FEBRUARY 24, 2021
For many daily insulin users, the experience is an important one, as it is directly related to quality of life and medication compliance. The injection experience is an often-overlooked aspect in insulin administration. We spend a lot of time trying to understand the human elements associated with it.

The Pharma Data
NOVEMBER 4, 2020
His broad leadership experience in the pharmaceutical industry, from intellectual property and regulatory compliance to product manufacturing and distribution, presents an exciting opportunity for XPhyto,” said Hugh Rogers, CEO of XPhyto. Thoresen is an important addition to XPhyto’s board of directors. XPhyto Therapeutics Corp.
Delveinsight
NOVEMBER 26, 2020
Non-adherence and non-compliance with the present treatment regimen is another huge hurdle in the road. Consequently, the growth of the RVO drug market is driven by the deep penetration of the emerging pipeline therapies , the rising geriatric population, and an increase in disease awareness.

The Pharma Data
JANUARY 18, 2021
Utilizing drug delivery platform technologies, BetterLife is refining and developing drug candidates from a broad set of complementary interferon-based technologies which have the potential to engage the immune system to fight virus infections, such as the coronavirus disease (COVID-19) and human papillomavirus (HPV).

The Pharma Data
DECEMBER 14, 2020
is an emerging biotechnology company engaged in the development and commercialization of therapeutic pharmaceuticals as well as drug delivery platform technologies. About BetterLife Pharma Inc. BetterLife Pharma Inc. For further information please visit www.abetterlifephama.com. About Transcend Biodynamics.

XTalks
NOVEMBER 6, 2024
Gelteq centers its technology on a proprietary, customizable gel platform designed for precise drug delivery, supporting prescription drugs, nutraceuticals, pet care and sports supplements. Priced at $4.0

The Pharma Data
JANUARY 27, 2021
Utilizing drug delivery platform technologies, BetterLife is refining and developing drug candidates from a broad set of complementary interferon-based technologies which have the potential to engage the immune system to fight virus infections, such as the coronavirus disease (COVID-19) and human papillomavirus.

Roots Analysis
SEPTEMBER 13, 2023
Conjugation with a Variety of Molecular Conjugates and Labels The combination of the conjugates / labels with molecules ( proteins, peptides, antibodies and small molecules ) is being used abundantly for several applications, ranging from therapeutics and diagnostics to isolation and characterization of drug delivery systems.

The Pharma Data
JANUARY 3, 2021
The Company’s pipeline leverages its proprietary bioerodible Durasert® technology for extended intraocular drug delivery including EYP-1901, a potential six-month sustained delivery intravitreal anti-VEGF treatment initially targeting wet age-related macular degeneration.

Pharmaceutical Technology
JUNE 23, 2022
This challenging environment, in addition to the measures taken to restrict people’s movement in order to reduce the viruses spread, led to many of the vital clinical trials being carried out to shift towards decentralised trial procedures that utilised virtual medicine and direct-to-patient (DtP) drug delivery.

pharmaphorum
NOVEMBER 17, 2021
SMi presents the Inaugural: Transdermal and Microneedle Drug Delivery Conference. Latest innovations in drug delivery beyond the needle. SMi is proud to present the Transdermal and Microneedle Drug Delivery Conference, taking place on the 24th to 25th January 2022, in London. pharmaphorum . pharmaphorum .

Delveinsight
JANUARY 22, 2021
There are newer agents coming to the market, for instance, Fc receptor via monoclonal IgG antibodies mechanism, BTK [Bruton tyrosine kinase] inhibition, and so on, however, the real challenge lies in the compliance in terms of patients perspectives such as cost and tolerance.

Roots Analysis
SEPTEMBER 22, 2023
These innovative instruments combine precision, convenience, and patient-centered design to revolutionize drug delivery. These devices are equipped with advanced features like programmable dosing, connectivity to mobile apps, and safety mechanisms to ensure accurate and controlled drug delivery.

XTalks
MAY 23, 2024
Dr. Bokhari: Medicus Pharma, through its subsidiary SkinJect, addresses these needs with its innovative transdermal drug delivery system (TDDS). The D-MNA delivers the drug directly to the tumor site, enhancing effectiveness while ensuring there are no systemic side effects. How is Medicus Pharma innovating in this space?

pharmaphorum
JUNE 22, 2021
GlaxoSmithKline (GSK) has reported encouraging data for its COVID-19 antibody sotrovimab, and an alliance with Halozyme to develop a new generation of long-acting HIV drugs, as it prepares to give a much-anticipated update to shareholders tomorrow.

The Pharma Data
JANUARY 16, 2021
Since its launch at the end of 2018, Etta’s X-Porator F1 has been accepted and adopted by many Chinese antibody drug and IVD benchmark companies, bringing significant benefits to customers. About JS Bio.
Roots Analysis
MARCH 9, 2022
1] The desired function of excipient in formulation of the medicinal product is to carry the active component to the target site with an active contribution in improving its biological effect, stability, appearance and patient compliance. [2]. Biopharmaceutical Excipient Manufacturing Market.
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?


Let's personalize your content