Pharma packaging solutions, design and companies
Pharmaceutical Technology
JUNE 25, 2024
Explore the critical aspects of pharma packaging solutions, design, and companies. What to look for to ensure product safety and compliance.
This site uses cookies to improve your experience. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country, we will assume you are from the United States. Select your Cookie Settings or view our Privacy Policy and Terms of Use.
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.
Used for the proper function of the website
Used for monitoring website traffic and interactions
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.

Pharmaceutical Technology
JUNE 25, 2024
Explore the critical aspects of pharma packaging solutions, design, and companies. What to look for to ensure product safety and compliance.

Pharmaceutical Technology
JUNE 14, 2023
Navigating the complexities of pharma and biotech packaging services can be difficult. With the growing number of complex therapies that require specialized packaging and handling requirements, selecting the right contract packaging organization (CPO) involves evaluating what services and additional benefits they can bring to your business.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Pharmaceutical Technology
SEPTEMBER 15, 2022
Packaging plays a vital role in maintaining the quality, safety, user-friendliness and marketability of drugs and other pharmaceutical products. Finding the best commercial packaging suppliers in contract marketing. Pharmaceutical packaging formats and materials. Pharmaceutical packaging formats and materials.

XTalks
MAY 13, 2022
A container closure system consists of all the packaging components that contain and protect a pharmaceutical product. They provide specific guidance on packaging requirements for closed closure integrity (CCI) and general integrity testing. Container Closure Systems. Container closure systems are highly regulated by health agencies.

Pharma Packaging Solutions
NOVEMBER 4, 2024
In the complex world of pharmaceutical packaging, selecting the right blister packaging supplier is a critical decision that can significantly impact product presentation, protection, and compliance. Material Selection Central to the blister packaging process is the selection of appropriate materials.

XTalks
MAY 5, 2022
Related: Will An Upcycled Certification Mark on Packaged Foods Resonate With Consumers? Food packaging labels are branching beyond traditional categories of organic and fair trade given the rising interest among consumers for easily discernible product information. billion by 2027, growing at a compound annual growth rate (CAGR) of 3.6
Pharma Packaging Solutions
OCTOBER 29, 2024
As a premier provider of medical device packaging solutions, we recognize the prime importance of adhering to global regulations while creating packaging solutions that protect both products and patients. International Standards and Classifications Compliance doesn’t end with the EU GMP certifications.

Pharma Packaging Solutions
NOVEMBER 8, 2024
Medical device packaging plays a key role in ensuring the safety, efficacy, and integrity of medical devices from the point of manufacture to the end-user. At Tjoapack, we are a premier provider of top-tier medical device packaging services. The packaging must adhere to guidelines set by authorities such as the FDA and EU GMP.

Pharma Packaging Solutions
NOVEMBER 1, 2024
At Tjoapack, we are your premier provider of private label pharmaceutical packaging solutions. Impact on Packaging The increase in private label pharmaceuticals has had a significant effect on packaging. Consequently, packaging solutions must take a delicate balance between quality and cost-effectiveness.

World of DTC Marketing
MARCH 9, 2021
eMail represents an opportunity for pharmaceutical companies to get closer to their customers while increasing adherence and compliance. The brand, for example, is receiving complaints on packaging that’s hard to open. The key to effective email marketing is segmentation and personalization.

XTalks
NOVEMBER 6, 2024
XTALKS WEBINAR: Unlock Growth in Medical Device Manufacturing with an AI-Powered eQMS Live and On-Demand: Monday, December 9, 2024 , at 11am EST ( 4pm GMT / UK) Register for this free webinar to discover how AI integration can transform compliance, streamline operations and position medical device manufacturers for unprecedented growth.
Pharma Packaging Solutions
JUNE 9, 2023
Quality medical device packaging is crucial for the health and well-being of countless people all around the world. With such an important job to do, it’s essential that the medical device packaging you choose for your product is up to the task. Here are a few factors to take into consideration when selecting medical device packaging.

Drug Patent Watch
FEBRUARY 19, 2025
In the context of generic drugs, QA involves a range of activities, including: Conducting regular audits and inspections of manufacturing facilities to ensure compliance with regulatory requirements Testing finished products for purity, potency, and stability Verifying the accuracy of labeling and packaging Monitoring the supply chain to prevent contamination (..)

Pharma Packaging Solutions
SEPTEMBER 8, 2022
That’s why the drug packaging process is so important, and must be carried out correctly. Primary packaging is the first step in the drug packaging process. Packing materials that come in direct contact with pharmaceutical products—such as blister packs, bottles, vials, and ampules—are all examples of primary packaging.

Pharma Packaging Solutions
SEPTEMBER 15, 2022
Pharmaceutical packaging companies play a critical role in the pharmaceutical industry. Thus, choosing a packaging company to partner with is a very important decision. When evaluating a pharmaceutical packaging company, your first order of business should be determining whether it has the capabilities to meet your specific needs.

Pharma Packaging Solutions
AUGUST 23, 2022
Pharmaceutical packaging plays a critical role in the medical supply chain. There are several different types of pharmaceutical packaging, each with its own purpose. . Primary Pharmaceutical Packaging. There are several different types of pharmaceutical packaging, each with its own purpose. .
XTalks
JANUARY 29, 2025
This initiative focuses on enhancing infrastructure to support the clinical and commercial-scale final assembly and packaging of drug-device combination products, emphasizing advanced drug delivery systems, particularly injectable formats.

Roots Analysis
APRIL 20, 2022
This continuously growing pipeline of pharmaceutical drug products has inadvertently led to an increase in the demand for their associated primary packaging and Secondary Packaging solutions. . 3] Some of the advantages offered by pharmaceutical secondary packaging have been depicted below. Company Competitiveness Analysis.

Pharmaceutical Technology
SEPTEMBER 5, 2022
Quality in terms of number of defective items, packaging and labelling, quality management system certification, research, development, and innovation. Some of the major factors considered in the selection of a wholesaler include: · Cost that includes product type, payment terms, and delivery cost. Geographical location for timely delivery.

XTalks
JULY 26, 2024
They ensure efficiency, compliance and profitability. Wherefour , a leader in ERP solutions, is transforming and simplifying how food and beverage companies manage production and compliance. They help manage complex operations, track inventory and ensure regulatory compliance.

FDA Law Blog
JULY 22, 2024
The American Conference Institute (“ACI”) is holding its 2nd West Coast Editionof its Legal, Regulatory, and Compliance Forum on Cosmetics & Personal Care Products from September 25-26 at the Le Meridien Delfina, Santa Monica, California. s John W.M. s John W.M.

Advarra
SEPTEMBER 8, 2022
After platform review, if a decision is made to validate, staff should generate documentation to ensure Part 11 compliance. As the first requirement in Part 11 compliance, validation is systematic documentation for a system’s requirements. This review can be documented in a vendor’s audit or within your own validation package.

Pharmaceutical Commerce
JANUARY 31, 2024
Under the DSCSA law, wholesale distributors, re-packagers, dispensers, and other third-party logistics providers must implement interoperable and electronic tracing of prescription products at the package level.

FDA Law Blog
MARCH 2, 2022
By Riëtte van Laack — On February 18, 2022, the US Consumer Product Safety Commission’s (CPSC) Office of Compliance and Field Operations issued a guidance for household substances not intended for household use under the Poison Prevention Packaging Act (PPPA). No, that is not a typo.

Cloudbyz
MARCH 3, 2023
Regulatory Compliance Compliance with regulatory requirements is critical for CROs. This can make it challenging to ensure compliance with the latest regulations and guidelines. Larger CROs may offer more attractive compensation packages and career advancement opportunities.
Cloudbyz
AUGUST 19, 2024
Compliance with their regulations is mandatory for manufacturers seeking to market their devices globally. Regulatory compliance: Labels must comply with the regulations of the country in which the device is marketed, including language requirements, symbols, and content.

Roots Analysis
FEBRUARY 1, 2024
Further, the cold chain storage process involves the cold chain storage, cold chain transportation, and monitoring temperature controlled packaging products. Therefore, the pharmaceutical, biotech, food and chemical industries usually rely on cold chain packaging for the transportation of their products.
Pharmaceutical Technology
AUGUST 31, 2022
The high-quality water supplied by ILC Dover offers global compliance by meeting pharmacopeial monograph parameters for authorities across the world, including US Pharmacopeia (USP), European Pharmacopoeia (Ph. and Japanese Pharmacopoeia (JP).

Pharma Packaging Solutions
NOVEMBER 11, 2024
By providing all required components in one package, medical device kitting reduces the duration of procedures and improves overall productivity. Medical device kitting promotes standardization by packaging specific components together, ensuring the same tools and supplies are used across different procedures or facilities.

Camargo
NOVEMBER 15, 2021
Batch steps include filter drying, milling, and packaging. Proposals must demonstrate the potential public health benefit and the excipient’s characteristics that may lead to new drug development, as well as the ability of the manufacturer to submit a complete safety package in the required time frame for Stage Two evaluation.

XTalks
JULY 19, 2024
The term “daily value” is central to understanding the Nutrition Facts label, a staple on nearly every packaged food product in the US since 1994. Packaging also plays a role. The history and implementation of the Nutrition Facts label are more complex than they appear.
Cloudbyz
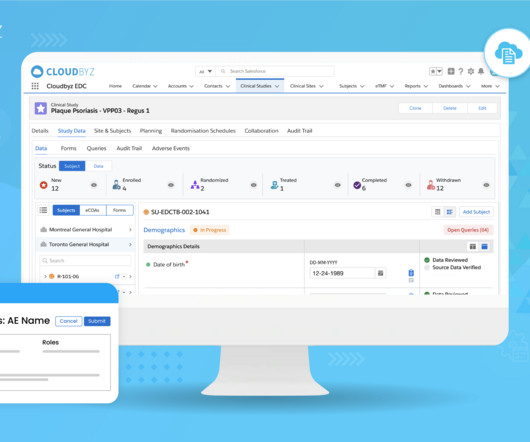
JUNE 21, 2023
Utilize SDTM Conversion Tools: To convert the extracted data into SDTM format, employ SDTM conversion tools or software packages available in the market. Employ CDISC’s validation tools, such as OpenCDISC Validator or Pinnacle 21 Enterprise, to verify compliance with CDISC SDTM rules and guidelines.

ACRP blog
JULY 26, 2023
That session is available as part of a Regulatory & Compliance replay package of conference sessions or in the Full Program replay package.] Edited by Gary Cramer The post Building Upon the Unique Perspective of Patient Advocacy Groups appeared first on ACRP.

BioTech 365
DECEMBER 16, 2020
iA Announces Reseller Agreement with BD iA Announces Reseller Agreement with BD iA’s Symphony Software platform will integrate the BD Rowa™ Pouch Packaging Solution, expanding iA’s solutions offering to include compliance packaging JOHNSON CITY, N.Y.–(BUSINESS

Pharma Marketing Network
AUGUST 9, 2023
Navigating these regulatory challenges is essential to ensure compliance, maintain trust, and effectively communicate the benefits and risks of pharmaceutical products. Adhere to Labeling and Packaging Requirements The labeling and packaging of pharmaceutical products are tightly regulated to ensure safety and accurate information.
Cloudbyz
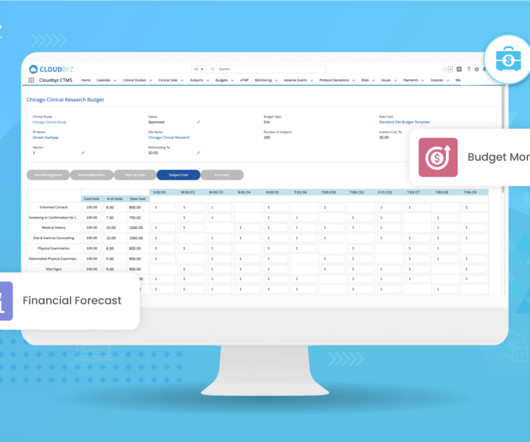
MAY 11, 2023
Investigational Product and Comparator Costs The costs of manufacturing, packaging, labeling, and shipping investigational products and comparators can add significantly to clinical trial expenses. These costs may also involve audits, inspections, and any corrective actions required to address compliance issues.

pharmaphorum
SEPTEMBER 15, 2022
It had been held up in the US by compliance issues that forced Mallinckrodt to change its packaging and labelling manufacturing facility and resulted in the FDA rejecting its application in February.

pharmaphorum
OCTOBER 12, 2022
Primary Packaging Development. Delve into the considerations for material components for the development of primary packaging. Kevin Kusmierek, Scientist, Primary Packaging and Medical Devices, CSL Behring. Michael Becker, Packaging Engineer, Boehringer Ingelheim. Sustainability for Injectable Delivery Devices.

FDA Law Blog
MARCH 27, 2023
Such codes need to be placed on device labels and packages to allow devices to be easily identified and tracked throughout their lifecycle, except where the rule provided for an exception or alternative. The compliance dates were first published in 2013, and subsequently updated in various guidance documents and regulations published by FDA.

FDA Law Blog
OCTOBER 31, 2021
Under SB 343, a product or packaging is not recyclable in California if: It includes components, inks, dyes, adhesives, or labels that prevent its recyclability; It contains intentionally added chemicals identified pursuant to regulations implementing section 42370.2(g)(4) g)(4) of the California Public Resources Code; or.

XTalks
AUGUST 15, 2024
Despite concerns, current evidence does not show significant migration of these particles from plastic food packaging into foods and beverages. They enhance the ability to trace the history, location and application of items, which is vital for quality control and regulatory compliance.

ACRP blog
JANUARY 11, 2024
As the saying goes, “With great power comes great responsibility,” and with so many clinical study site leaders now taking technological powers into their hands that were traditionally held by sponsors, some of them and their staff may be surprised to find the awesome responsibilities that come packaged with the deal.

pharmaphorum
NOVEMBER 24, 2022
And even once the facility is fit for purpose, specialised staff are required to maintain compliance with strict regulations. For example, a new pharma firm may work with a COD provider on a small product run, developing a modular cleanroom environment at just 500 square feet, divided into two rooms – for product development and packaging.

pharmaphorum
MAY 27, 2022
The possibilities are endless, and in the context of trial participation and access to medicine technologies could assist: Reduced assessment times and hence increased patient compliance. As a Non-Clinical Assessor at MHRA he assessed non-clinical data packages for new and generic medicines. About the interviewees.
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?


Let's personalize your content