Navigating Regulatory Compliance in the Pharmaceutical Industry
Pharma Mirror
NOVEMBER 7, 2023
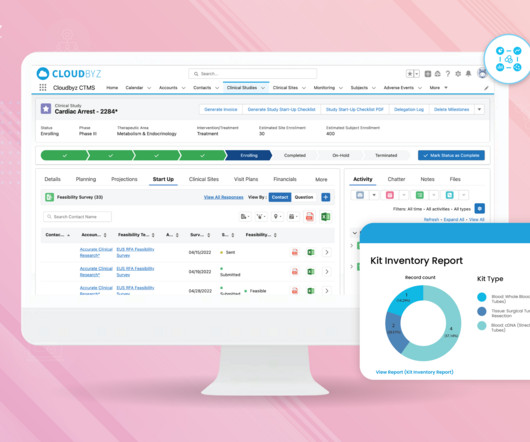
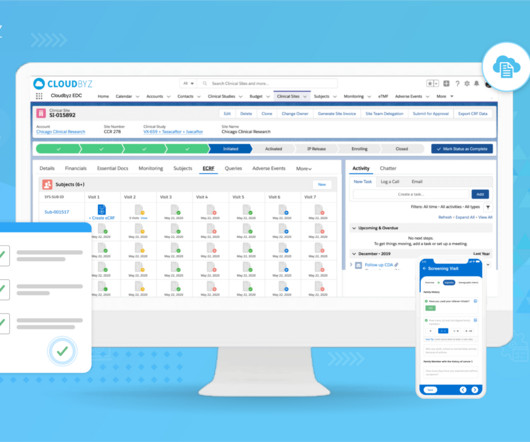
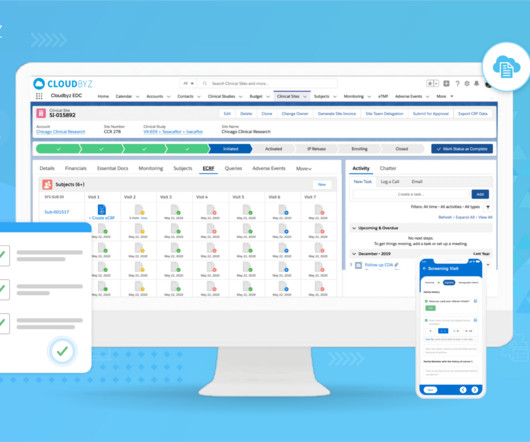
Navigating regulatory compliance in the pharmaceutical industry is crucial to ensure the safety, efficacy, and quality of pharmaceutical products. The pharmaceutical industry is heavily regulated in most countries to protect public health and to maintain the integrity of the products.











































Let's personalize your content