Container Closure Integrity Testing: A Necessity for Pharmaceutical Industry
Roots Analysis
FEBRUARY 28, 2022
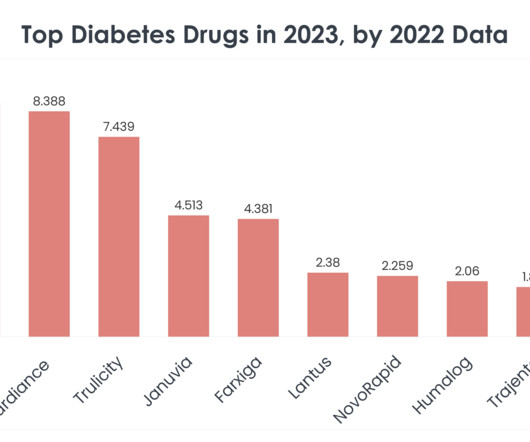
Considering patient’s health and safety, the drug products are expected to be free from microbial contamination and should be safe for use throughout the drug’s life cycle. One of the stages that require quality assurance is packaging of the drug product, as compromised packaging could have serious consequences.












































Let's personalize your content