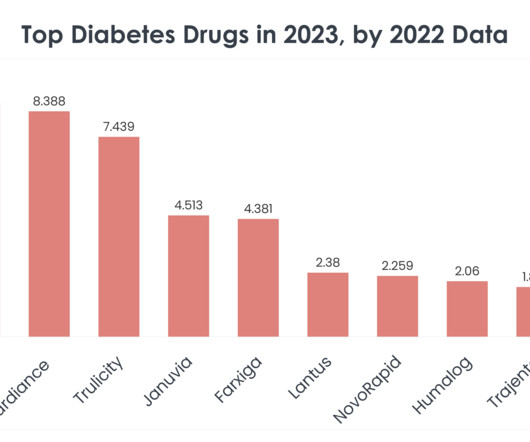
Top 15 Diabetes Drugs in 2023 by 2022 Sales Statistics
XTalks
FEBRUARY 8, 2024
Diabetes remains a critical global challenge, affecting millions of lives and commanding significant attention from the medical, life sciences and pharmaceutical sectors. In 2022, Januvia was flagged for nitrosamine contamination , a potential carcinogen commonly produced as a byproduct during drug manufacturing.








Let's personalize your content