Biologic Therapeutics Development, Part 1: Definition and Distinct Characteristics
Camargo
NOVEMBER 29, 2021
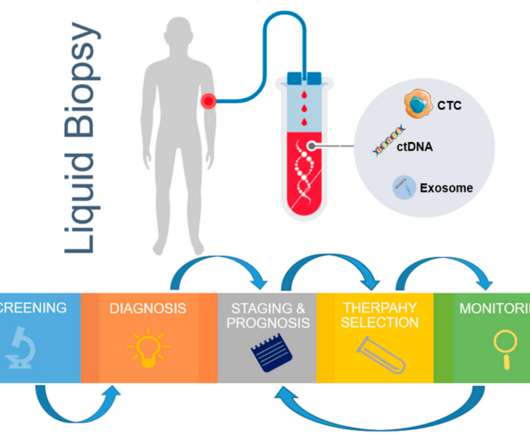
Essentially, a biologic is a product that is produced from living organisms or that contains components of living organisms. They are often heat sensitive, membrane impermeable, subject to enzymatic degradation, and susceptible to microbial contamination (a factor which impacts manufacturing steps and quality considerations).












Let's personalize your content