How API CMOs can improve containment capabilities without facility acquisition
Pharmaceutical Technology
NOVEMBER 17, 2022
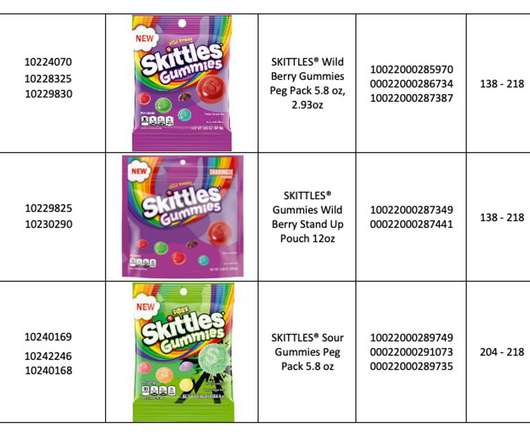
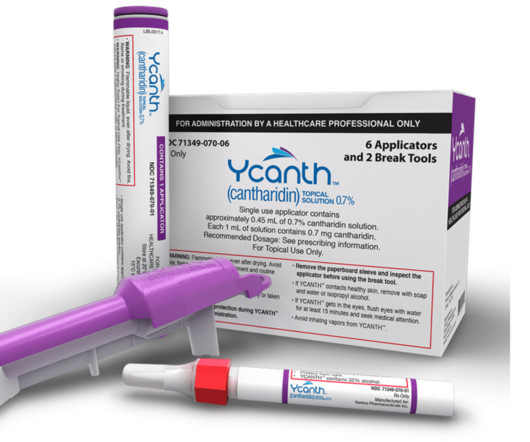
According to GlobalData analysis, specialist small molecule API capabilities such as containment or controlled substances are driving large contract manufacturing organisations (CMOs) to acquire facilities. Flexible containment solutions for potent APIs. Partnering with containment experts.
















































Let's personalize your content