Phesi’s AI-driven Trial Accelerator platform contains over 100 million patients
Pharma Times
FEBRUARY 12, 2024
The platform delivers digitalised patient data to improve clinical trials and development
This site uses cookies to improve your experience. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country, we will assume you are from the United States. Select your Cookie Settings or view our Privacy Policy and Terms of Use.
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.
Used for the proper function of the website
Used for monitoring website traffic and interactions
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.

Pharma Times
FEBRUARY 12, 2024
The platform delivers digitalised patient data to improve clinical trials and development
Pharmaceutical Technology
DECEMBER 12, 2022
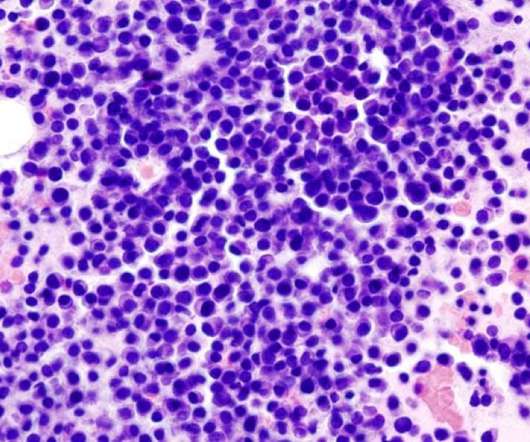
Gilead company Kite has entered an international strategic partnership with Arcellx for the joint development and commercialisation of the latter’s T-cell therapy, CART-ddBCMA, to treat relapsed or refractory multiple myeloma patients. Additionally, Kite will make other potential payments to Arcellx.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
Pharmaceutical Technology
FEBRUARY 8, 2023
On February 7, at a town hall organised to discuss clinical trial designs for gene therapies, FDA experts pushed pharma players to look for ways to establish clinical effectiveness despite the challenges in recruiting patients with rare diseases.

Rethinking Clinical Trials
OCTOBER 2, 2023
The Patient-Centered Outcomes Core has developed a new tool kit to provide resources to support the capture of patient-reported outcome (PRO) measures in diverse study populations. The post October 2, 2023: Patient-Centered Outcomes Core Develops Tool Kit to Promote Health Equity in PROs appeared first on Rethinking Clinical Trials.
Pharmaceutical Technology
MAY 24, 2023
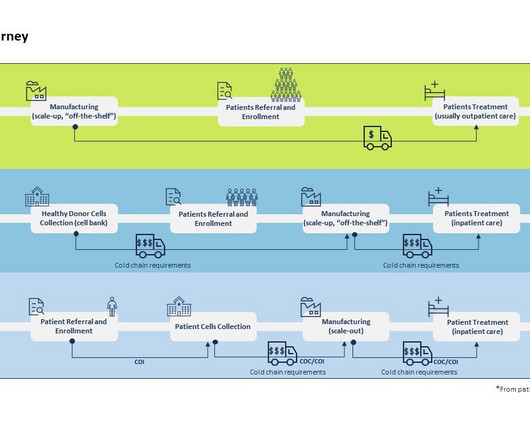
By Luisa Sterkel & Joana Loureiro , Tenthpin Consultants The promise and potential of cell and gene therapies (CGT) has emerged in the recent past and currently over 1.500 CGT are registered for clinical trials holding great hope for the treatment of challenging and uncurable diseases.

Camargo
NOVEMBER 11, 2020
Each month, Camargo’s “In the News” series highlights important changes and advancements in the regulatory and development space and explores how those changes could impact your program. Still, navigating such patent issues during drug development can be difficult, and Camargo can assist in finding a safe course. Ken Phelps.

Camargo
OCTOBER 14, 2021
Each month, Camargo’s “In the News” series highlights important changes and advancements in the regulatory and development space and explores how those changes could impact your program. Continued development of the use of complex innovative trial designs. Continue development of the use of Real-World Evidence.
Pharmaceutical Technology
OCTOBER 6, 2022
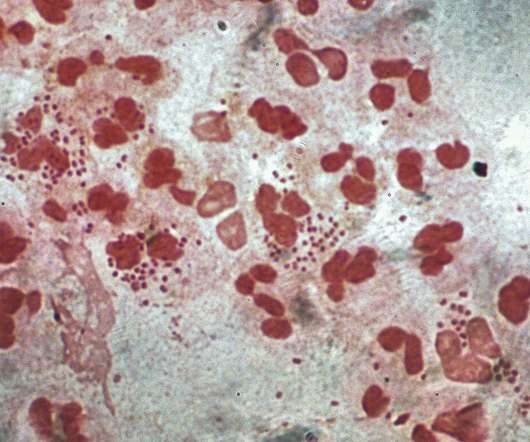
from the US National Institutes of Health (NIH) unit National Institute of Allergy and Infectious Diseases (NIAID) to develop a prophylactic intranasal vaccine against Neisseria gonorrhoeae (NG). Leveraging its outer membrane vesicles (OMV) platform technology, Intravacc will develop the vaccine.

Camargo
DECEMBER 10, 2021
Each month, Camargo’s “In the News” series highlights important changes and advancements in the regulatory and development space and explores how those changes could impact your program. This means that full approval can only come from a clinical trial that assesses adult height. Voxzogo also received a priority review voucher.

pharmaphorum
JANUARY 14, 2022
The European Commission, EMA and national regulators within the EU have launched an initiative to change the way clinical trials are designed and run in order to position the bloc as an international “focal point” for clinical research.

Camargo
JANUARY 14, 2021
Each month, Camargo’s “In the News” series highlights important changes and advancements in the regulatory and development space and explores how those changes could impact your program. If you are developing a combination product, Camargo can help you design the fastest, most efficient path to approval. Co-Authors: Ken Phelps.

Worldwide Clinical Trials
AUGUST 29, 2024
By: Nathan Chadwick, Therapeutic Strategy Lead, Rare Disease Over the last few years in clinical trials, particularly within the rare disease community, a notable shift is underway, where patients and caregivers are taking the lead in reaching out to clinical trial sites rather than the other way around.

Camargo
DECEMBER 13, 2021
The development of biological products (or biologics) represents a major advancement in modern medicine, enabling the treatment of patients with many illnesses where no other therapeutics were previously available. Regulatory Considerations for Biologics. Section 351(a) is the traditional pathway for approving biologics under the PHS Act.

Pharmaceutical Technology
AUGUST 24, 2022
Patients provide blood samples for clinical trial sponsors to develop and deliver these emerging immunotherapies. Each sample they give is precious, as they all provide a wealth of biological data that contributes to therapy development. More complex panels provide information for exploratory assessment in clinical trials.

pharmaphorum
NOVEMBER 28, 2021
An investigator-led trial of Jazz Pharma’s cannabis extract-based drug Sativex in glioblastoma – an aggressive form of brain cancer – will get underway in the UK next year. billion takeover of Sativex’ developer GW Pharma earlier this year. Jazz acquired rights to Sativex when it completed its $7.2
Pharmaceutical Technology
JUNE 9, 2023
VEVYE, the development name of which is CyclASol, is a cyclosporine, solubilised solution in a new, water-free excipient. CyclASol is topical anti-inflammatory and immunomodulating ophthalmic drug solution containing 0.1%

Pharmaceutical Technology
AUGUST 3, 2022
The potential to use these scientific breakthroughs to make a real difference to patients’ lives is what drives many highly skilled scientists to the world of drug development. This is where the process development stage, which directly follows the drug discovery and early formulation work, can make or break a drug’s success.

Camargo
APRIL 20, 2021
This trend progressively reduces the attractiveness of engaging in the early stages of oncology therapy development in the eyes of Big Pharma and opens the space for small pharma and biotech players. Become Fluent in the Drug Development “Languages.”. The language of accounting, to optimally allocate capital to maximize results.
pharmaphorum
SEPTEMBER 14, 2020
AstraZeneca has resumed UK trials for its coronavirus vaccine, after the country’s medicines regulator gave the all-clear following a safety scare. A UK safety committee has concluded its investigations and recommended to the country’s Medicines and Healthcare products Regulatory Agency (MHRA) that trials are safe to resume.

pharmaphorum
DECEMBER 4, 2022
Clinical trials of a wireless brain chip developed by Elon Muck’s Neuralink company will be tested in human volunteers within the next six months – and Musk himself says he intends to have one implanted for a future demo event. The post Elon Musk’s Neuralink brain interface chip set for human trials appeared first on.

World of DTC Marketing
NOVEMBER 27, 2020
Pfizer and Moderna have provided data from their large-scale Phase 3 trials only via news releases , which contained the highly promising news that both vaccines were 90 percent effective or more and have not presented any serious safety concerns. The results of trials were announced in press releases rather than peer-reviewed papers.

Pharmaceutical Technology
APRIL 28, 2023
It has also been approved by the FDA for otitis media prevention in infants and children aged between six weeks and five years caused by the original seven serotypes contained in PREVNAR. The latest approval is based on data obtained from the Phase II and Phase III trials of PREVNAR 20 for paediatric indication.

Worldwide Clinical Trials
FEBRUARY 21, 2024
During clinical development, new chemical entities (NCEs) require an absorption, metabolism, and excretion (AME) study. Regardless of the formulation, the entire dose must be administered to each subject, and the dosing containers must be checked for residual radioactivity.
Pharmaceutical Technology
FEBRUARY 26, 2023
Finch Therapeutics’s Phase III PRISM4 trial for recurrent Clostridioides difficile infection (CDI) and MaaT Pharma’s Phase III trial in steroid-resistant acute graft-versus-host disease were both put on hold. While the French MaaT Pharma has submitted further information to the FDA, its trial remains on hold. with placebo.
pharmaphorum
AUGUST 6, 2021
Psychiatric disorders in particular represent a trial area with significantly high drop-out rates and poor patient recruitment. Despite this, new strategies devised over the last five years are beginning to reverse the trend, and both reduce stigma and increase awareness to funnel new patients into these important trials. .

XTalks
NOVEMBER 29, 2022
Xtalks interviewed Devon Adams to learn more about his work related to decentralized oncology trials as a senior analyst for legislative policy specific to clinical trials at the ACS CAN. Read on to learn more! Over 1,100 cancer patients and cancer survivors responded.

Pharmaceutical Technology
JANUARY 24, 2023
Developed with the University of Edinburgh, the EXACT self-contained technology enables MeCP2 protein therapeutic levels while avoiding overexpression-related toxicities. Neurogene stated that the FDA IND clearance allows it to commence a Phase II/II trial of NGN-401 in female paediatric Rett syndrome patients.

Camargo
SEPTEMBER 14, 2021
As regulatory requirements become increasingly harmonized across the globe, the development and marketing of pharmaceutical products worldwide are also becoming more streamlined. INDs must contain full study reports of nonclinical and clinical (if available) studies.
Pharmaceutical Technology
JUNE 21, 2023
This followed positive results from CSL’s Phase III HOPE-B trial (NCT03569891). Hemgenix works by dosing a patient with an engineered adeno-associated virus (AAV), containing the gene responsible for producing a protein called factor IX. Once administered, the body will then be able to produce factor IX and prevent severe bleeds.

XTalks
APRIL 29, 2022
Eli Lilly announced that its obesity drug tirzepatide has scored favorably in a late-stage clinical trial, with results showing that people who took the drug lost an average of 50 pounds, or 21 percent, of their body weight compared to placebo. GLP-1 agonists were first developed as diabetes treatments. percent and 22.5

Pharmaceutical Technology
SEPTEMBER 14, 2022
Successful TIL therapy depends on the infusion product containing tumour-reactive T cells that, on infusion, generate an anti-tumour immune response that causes disease regression. While this approach is in the early stages of development, it is an exciting avenue of research.

Pharmaceutical Technology
APRIL 4, 2023
KEYTRUDA is an anti-PD-1 therapy developed by Merck, while Padcev has been developed by Astellas and Seagen. The combination therapy can be used to treat la/mUC patients who do not qualify for cisplatin-containing chemotherapy. The trial was jointly conducted by Seagen and Astellas.
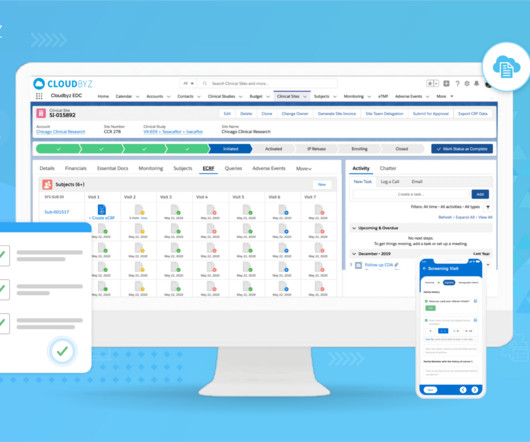
Cloudbyz
JUNE 16, 2023
Clinical trials are crucial for advancing medical research and developing innovative treatments. Effective clinical trial data archiving is essential to ensure data integrity, regulatory compliance, and seamless access. Data Complexity: Clinical trial data often comes in diverse formats, such as text, images, audio, and video.

Pharmaceutical Technology
JANUARY 31, 2023
It contains a single mRNA sequence encoding for a stabilised prefusion F glycoprotein. Moderna’s mRNA platform has now demonstrated two positive Phase III infectious disease trial results and we continue to advance a portfolio of respiratory mRNA vaccines targeting the most serious diseases.” The findings showed 83.7%
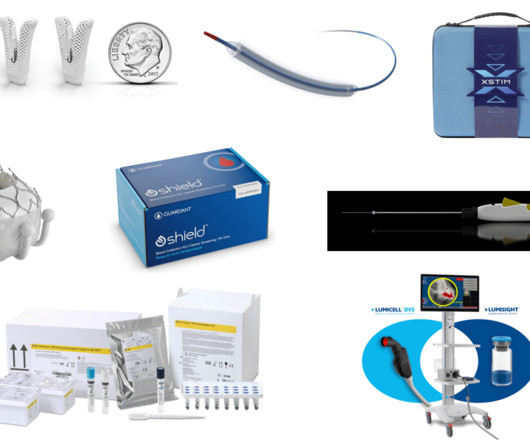
XTalks
JANUARY 21, 2025
TriClip G4 System Manufacturer/developer : Abbott Medical Date of FDA approval : April 1, 2024 Approved for : Tricuspid regurgitation (TR). Abbott Spinal Cord Stimulation (SCS) Systems Manufacturer/developer : Abbott Medical Date of FDA approval : May 30, 2024 Approved for : Chronic, hard-to-manage pain in the torso, arms and legs.

Pharmaceutical Technology
SEPTEMBER 20, 2022
Unlike commercial pharmaceutical packaging, the primary consideration in clinical trial packaging is protecting the product quality and reliability for research. Finding the best clinical trial packaging services providers. Clinical trial packaging and labelling solutions. Clinical trial packaging and labelling solutions.

FDA Law Blog
MARCH 7, 2024
Clissold — The trio of CDER, CBER, and CDRH released a new draft guidance titled “ Use of Data Monitoring Committees in Clinical Trials ” that revises the 2006 guidance “Establishment and Operation of Clinical Trial Data Monitoring Committees” and, when final, will replace the 2006 guidance.

Delveinsight
JULY 27, 2021
Remarkable drop was observed in altered filamin A plasma levels after treatment with simufilam in Phase 2b trials. a biotechnology (clinical stage immuno-oncology) company from California publicized the design of its new XPro therapy in Phase 2 clinical trials. P-tau 181 plasma levels were also reduced in a good amount. gingivalis.

Pharmaceutical Technology
SEPTEMBER 14, 2022
Pharmaceutical drug research and development (R&D) activities are capital-intensive, which makes the outsourcing of clinical dose manufacturing and marketing popular. The download contains detailed information on the providers and their services and solutions, alongside contact details to aid your purchasing or hiring decision.

XTalks
MAY 18, 2023
The wireless charger — the transmitter — contains a metallic coil that converts electrical energy into a magnetic field, which is then collected and reverted into electricity by the receiver inside the implanted device. Resonant Link’s medical device chargers are undergoing animal trials this year.
pharmaphorum
SEPTEMBER 1, 2020
AstraZeneca has expanded development of COVID-19 vaccine AZD1222 into the US, beginning a phase 3 clinical trial across all adult age groups. The UK pharma said that the trial will recruit up to 30,000 adults aged 18 years or over to assess the safety, efficacy and immune response.

Worldwide Clinical Trials
JUNE 15, 2022
At the end of May, we hosted a webinar titled “ Changing Times, Changing Therapies: Keeping Up with Advancements in Cell and Gene Therapies ” to provide a quick update on the latest advancements and ongoing in development of these advanced therapeutics. We need to consider how to use diverse populations in gene therapy trials.

XTalks
NOVEMBER 6, 2020
Canadian clinical-stage biotech company Symvivo Corporation has developed an oral COVID-19 vaccine that entered clinical trials this week. The first healthy volunteer was dosed with the vaccine in Australia as part of the bacTRL-Spike COVID-19 Phase I clinical trial. COVID-19 Clinical Trials.

Pfizer
FEBRUARY 16, 2023
These study participants, representing approximately half of the total recruited participants in the trial, are being discontinued following violations of Good Clinical Practice (GCP) at certain clinical trial sites run by a third-party clinical trial site operator.
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?


Let's personalize your content