Liposome: A Novel Drug Delivery System
Roots Analysis
AUGUST 8, 2023
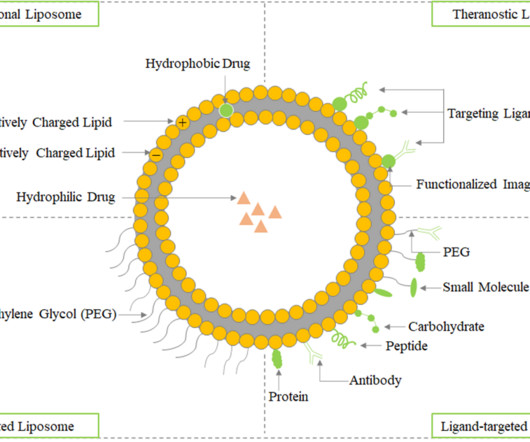
The phospholipid bilayers continuously surround the dispersing aqueous media and create a vesicular system in response to mechanical shaking or heating. The hydrophobic portion contains two fatty acid chains with 10-24 carbon atoms, while the hydrophilic part primarily consists of phosphoric acid linked to a water-soluble molecule.




















Let's personalize your content