Stevanto Group tackling flexible drug containment packages and filling solutions
Outsourcing Pharma
JANUARY 30, 2024
Two new offerings have been unveiled by drug delivery and solutions provider, Stevanto Group S.p.A.
This site uses cookies to improve your experience. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country, we will assume you are from the United States. Select your Cookie Settings or view our Privacy Policy and Terms of Use.
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.
Used for the proper function of the website
Used for monitoring website traffic and interactions
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.
Outsourcing Pharma
JANUARY 30, 2024
Two new offerings have been unveiled by drug delivery and solutions provider, Stevanto Group S.p.A.
Outsourcing Pharma
JANUARY 30, 2024
Two new offerings have been unveiled by drug delivery and solutions provider, Stevanto Group S.p.A.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
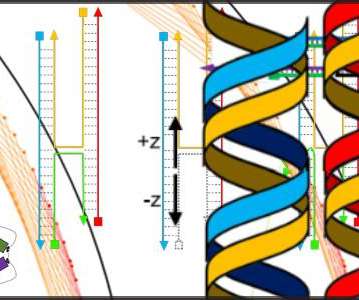
Scienmag
JANUARY 8, 2021
Dill/NIST In a technique known as DNA origami, researchers fold long strands of DNA over and over again to construct a variety of tiny 3D structures, including miniature biosensors and drug-delivery containers.

Roots Analysis
FEBRUARY 16, 2022
In earlier times, container closure integrity testing was not regarded as an essential step in the process of manufacturing. The company developing drug product through such an intense process, use to consider container closure system only for packaging of the product. [1] Container Closure Integrity Testing Services Market.

XTalks
JULY 26, 2024
These issues primarily stem from patients and healthcare providers miscalculating or incorrectly administering the drug. Compounded semaglutide products, unlike the US Food and Drug Administration (FDA)-approved ones, can vary widely in strength and packaging, making it easy for mistakes to happen.

Pharmaceutical Technology
FEBRUARY 27, 2023
According to GlobalData, Phase I drugs for Adrenal Insufficiency have a 67% phase transition success rate (PTSR) indication benchmark for progressing into Phase II. GlobalData’s report assesses how Hydrocortisone’s drug-specific PTSR and Likelihood of Approval (LoA) scores compare to the indication benchmarks.

Pharmaceutical Technology
FEBRUARY 3, 2023
In 2021, 72% of newly approved drugs were small molecules, and almost 50% of new drugs approved were OSD. While the industry is seeing advances in alternative drug delivery systems, oral solid doses, such as pills, capsules and soft gels, remain at the forefront of the industry.

Pharmaceutical Technology
SEPTEMBER 8, 2022
Pharmaceutical solid dosage forms are the most popular in the pharmaceutical industry and are one of the most used drug delivery methods across patient groups. The download contains detailed information on the providers and their services and solutions, alongside contact details to aid your purchasing or hiring decision.

Pharmaceutical Technology
SEPTEMBER 20, 2022
The information contained within the download document is intended for pharmaceutical manufacturers, wholesalers, retailers and distributors, pharmaceutical executives, medical representatives, business development managers, retail salesmen, sales managers, pharmacy executives, and any other individual involved in pharmaceutical marketing.

XTalks
JUNE 14, 2023
percent received approval from the US Food and Drug Administration (FDA) to treat dry eye disease. Attendees will understand best practices for novel drug delivery design and development. Attendees will understand best practices for novel drug delivery design and development. What Is Dry Eye Disease?

Pharmaceutical Technology
APRIL 16, 2023
Autoinjectors have revolutionised the convenient delivery of low-volume parenteral drugs. But with the advent of injection site absorption enhancers, drug delivery device innovations, as well as the recent primary container options, the practical subcutaneous self-administration of large-volume parenterals is becoming possible.

pharmaphorum
NOVEMBER 17, 2021
SMi Group’s 14 th Annual Conference and Exhibition: Pre-Filled Syringes and Injectable Drug Devices 2022 – 12 th – 13 th January 2022. Sustainability for Drug Devices Focus Day – 14 th January 2022. The Future of Drug Delivery and Combination Product Device Design.

FDA Law Blog
JUNE 13, 2024
Though some drug companies have been reluctant to delist certain patents from the Orange Book, the District Court of New Jersey just ordered Teva to delist 5 of its patents that it deemed improperly listed. The Court addressed Teva’s valid argument that the Inhaler Patents are drug product patents and thus listable.

XTalks
JANUARY 17, 2023
The US Food and Drug Administration (FDA) granted approval to AstraZeneca’s Airsupra (albuterol/budesonide), formerly known as PT027, for the as-needed treatment or prevention of bronchoconstriction and to reduce the risk of asthma attacks in individuals aged 18 years and older who have asthma.

XTalks
MAY 30, 2023
The patch itself is made of biodegradable nanocellulose and contains no plastic additives. The VTT Technical Research Centre of Finland has addressed this issue by developing a fully recyclable, modular ECG patch constructed from VTT’s novel cellulose e-skin material.

BioTech 365
JULY 7, 2021
the “Company”), a leading global provider of drug containment, drug delivery and diagnostic solutions to the … Continue reading →

BioTech 365
AUGUST 17, 2021
the “Company”), a leading global provider of drug containment, drug delivery and diagnostic solutions to the … Continue reading →

Pharmaceutical Technology
OCTOBER 26, 2022
Nano-based delivery systems are on the rise, as they enable manufacturers to deliver therapeutic agents to specific targeted tissue in a more controlled manner. Data indicates that the global nanopharmaceutical drugs market size reached USD 53.85 Billion in 2021 and is expected to reach USD 102.4 Billion in 2030.

XTalks
JULY 28, 2020
Excipients can take many forms, such as bulking agents, binders, coatings and colourants, and they are chosen based on their ability to aid in drug delivery, administration and even in pill identification and prevention of counterfeiting. But just how inert are these excipients?
STAT News
NOVEMBER 3, 2022
The companies allege Moderna’s vaccine uses their technology for a drug-delivery system without permission. Citing Genevant and Arbutus sued Moderna in February, seeking royalties from Moderna’s sale of its Covid-19 vaccine. Continue to STAT+ to read the full story…

The Pharma Data
JANUARY 27, 2021
Construction of commercial drug manufacturing facility in Germany. Psychedelic transdermal and sublingual drug formulation development. Vektor will position itself for commercial manufacturing, pipeline development and drug formulations for critical mental health conditions.

Roots Analysis
MAY 24, 2024
In the dynamic landscape of the healthcare sector, the evolution of drug delivery systems plays a significant role in transforming the management of chronic diseases. They are generally used in combination with primary containers, such as prefilled syringes and cartridges.

XTalks
MAY 18, 2023
The wireless charger — the transmitter — contains a metallic coil that converts electrical energy into a magnetic field, which is then collected and reverted into electricity by the receiver inside the implanted device. Our wireless charging for titanium cans changes all of that.” billion in 2022.
Druggist
OCTOBER 21, 2020
Terbinafine is recommended as the first-line oral antifungal drug. Topical products for toenail fungus treatment are recommended as the first option due to a better safety profile as compared to treatment with oral antifungal drugs. Amorolfine is classified as antimycotics drugs, which kills the fungus. Buy on Amazon Price incl.

The Pharma Data
DECEMBER 20, 2020
With this acquisition we are expanding our product pipeline to include psychedelic therapeutics, incorporating elements of our IP around drug delivery technology in which we already have prototypes developed, which we believe will propel us towards clinical studies relatively quickly. Ahmad Doroudian, CEO of BetterLife.

The Pharma Data
NOVEMBER 2, 2020
The US Food and Drug Administration has twice designated psilocybin (4-phosphoryloxy-N,N-dimethyltryptamine) a “breakthrough therapy” for the treatment of severe and treatment-resistant depression due to its potential to provide a major improvement over currently available therapeutics for an unmet medical need.

The Pharma Data
OCTOBER 27, 2020
On April 20, 2020, XPhyto announced a definitive development, technology purchase and licence agreement with 3a-Diagnostics for the development and commercialization of real-time, low-cost and easy-to-use screening tests using 3a’s pathogen specific biosensors and XPhyto’s oral dissolvable drug delivery platform.

The Pharma Data
JANUARY 31, 2021
(CSI ® ) (NASDAQ: CSII) announced today that it has partnered with Chansu Vascular Technologies, LLC (CVT) to develop novel peripheral and coronary everolimus drug-coated balloons (DCBs). Dr. Marco started working on drug delivery cardiovascular devices while in charge of Medical Affairs for Abbott Vascular where, following the Perclose Inc.

Druggist
NOVEMBER 19, 2020
Formoterol, which belongs to a group of drugs called long-acting beta2-agonist ( LABA ). As we already learned from the above combination inhaler in asthma contains two ingredients, one of which is inhaled corticosteroid and the other LABA. A spacer may offer drug delivery benefits in a wider patient group. Able Spacer®.

The Pharma Data
NOVEMBER 9, 2020
Dr. Löbenberg holds Health Canada licences for research and analytical testing of a wide range of psychedelic compounds under the Controlled Drugs and Substance Act and research and analytical testing licences for cannabis under the Cannabis Act. . DMT (N,N-dimethyltryptamine). Prof. Dr. Löbenberg. ” Prof.
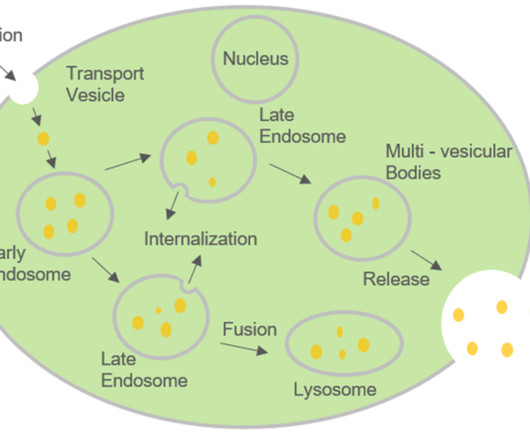
Roots Analysis
MARCH 8, 2023
Over time, various research studies have demonstrated the potential of exosomes ( membrane bound extracellular vesicles) in disease diagnosis, drug delivery and therapeutic applications. Targeted Drug Delivery: Ability to cross blood brain barrier cause lesser systemic effects and low toxicity.

FDA Law Blog
MARCH 12, 2024
Koblitz — After years of silence from FDA on whether certain patents could be listed in the Orange Book, some manufacturers of drug and device combination products have had a rude awakening lately.

Roots Analysis
JANUARY 17, 2024
They offer several advantages over traditional drug delivery systems ( such as vials and syringes ), including reduced chances of dosing errors, increased patient compliance and decreased risk of microbial contamination. However, this needle system does not contain a proper locking system, therefore, increased the chances of leakage.

The Pharma Data
DECEMBER 28, 2020
is an emerging biotechnology company engaged in the development and commercialization of therapeutic pharmaceuticals as well as drug delivery platform technologies. Forward-looking statements contained in this news release are expressly qualified by this cautionary statement. BetterLife Pharma Inc. Source link.

XTalks
AUGUST 30, 2022
The pharmaceutical industry deals with threats such as product liability, cyberthreats, supply chain disruptions, competition from generic drugs and more. Although these leadership styles are of very different natures, they also contain some commonalities. Therefore, strategic intent is vital when making decisions.

The Pharma Data
JANUARY 27, 2021
FDA Investigational New Drug (“IND”)-enabling pharmacology studies. About Eurofins Discovery: Eurofins Discovery, a business operating under the Eurofins BioPharma Services division, has supported drug discovery research for over 40 years. About BetterLife Pharma: BetterLife Pharma Inc. For more information, please visit: [link].

The Pharma Data
JANUARY 3, 2021
The strategic move to partition lung disease programs from TLC will allow TLC to maintain focus on its current pipeline of liposomal injectable formulation programs, such as TLC599 for osteoarthritis pain and TLC590 for postsurgical pain, while instilling to InspirMed funding designated for use in the development of inhalable drugs.

The Pharma Data
NOVEMBER 26, 2020
As drug molecules become more complex so do the options to deliver them. There are significant challenges in drug development in this space and we look forward to working closely with pharma and biotech partners to discover how we can add value to their programs, provide patient benefit and competitive product differentiation.”

The Pharma Data
JANUARY 25, 2021
” This announcement contains inside information for the purposes of Article 7 of Regulation (EU) 596/2014 (MAR). Midatech Pharma PLC (dual listed on LSE AIM: MTPH; and NASDAQ: MTP) isa drug delivery technology company focused on improving the bio-delivery and bio-distribution of medicines.

The Pharma Data
OCTOBER 20, 2020
” Mr. Bliss joins TLC following interim roles in cellular therapy, drug delivery and combination therapy where he led efforts to confirm research outcomes and partner the various technologies. This press release contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995.

Roots Analysis
JUNE 6, 2024
Autoinjector are medical devices that enable the rapid and safe delivery of drugs through different administration routes. Some of the advantages include convenient usability, enhanced precision, improved sterility, reduced risk of microbial contamination, easy storage and disposal, as well as quick delivery in emergency situations.

The Pharma Data
DECEMBER 14, 2020
While the medicalization of psychedelics is evolving and gaining momentum at an extraordinary pace, such therapeutics still face regulatory hurdles in the areas of manufacturing, formulating, and ultimately dispensing or the rescheduling of Schedule I drugs to patients. BetterLife Pharma Inc.

The Pharma Data
JANUARY 18, 2021
According to Health Canada, “the information currently available at the Controlled Substances Directorate, 2-bromo-LSD is NOT CONTROLLED under the Schedules to the Controlled Drugs and Substances Act.”. Forward-looking statements contained in this news release are expressly qualified by this cautionary statement. BetterLife Pharma Inc.

The Pharma Data
JANUARY 3, 2021
The Company focuses on reducing attrition in clinical trials and on enhancing drug molecules’ formulation performance through its nanoforming services. This press release contains forward-looking statements, including, without limitation, statements regarding Nanoform’s strategy, business plans and focus.
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?


Let's personalize your content