Stevanto Group tackling flexible drug containment packages and filling solutions
Outsourcing Pharma
JANUARY 30, 2024
Two new offerings have been unveiled by drug delivery and solutions provider, Stevanto Group S.p.A.
This site uses cookies to improve your experience. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country, we will assume you are from the United States. Select your Cookie Settings or view our Privacy Policy and Terms of Use.
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.
Used for the proper function of the website
Used for monitoring website traffic and interactions
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.
Outsourcing Pharma
JANUARY 30, 2024
Two new offerings have been unveiled by drug delivery and solutions provider, Stevanto Group S.p.A.

Pharmaceutical Technology
SEPTEMBER 8, 2022
Pharmaceutical solid dose manufacturing companies in contract marketing segment have gained a vital position in pharmaceutical formulations. Pharmaceutical solid dosage forms are the most popular in the pharmaceutical industry and are one of the most used drug delivery methods across patient groups.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Pharmaceutical Technology
OCTOBER 26, 2022
Nano-based delivery systems are on the rise, as they enable manufacturers to deliver therapeutic agents to specific targeted tissue in a more controlled manner. Data indicates that the global nanopharmaceutical drugs market size reached USD 53.85 Billion in 2021 and is expected to reach USD 102.4 Billion in 2030.

Roots Analysis
FEBRUARY 16, 2022
In earlier times, container closure integrity testing was not regarded as an essential step in the process of manufacturing. The company developing drug product through such an intense process, use to consider container closure system only for packaging of the product. [1] Our Social Media Platform. Web: [link].
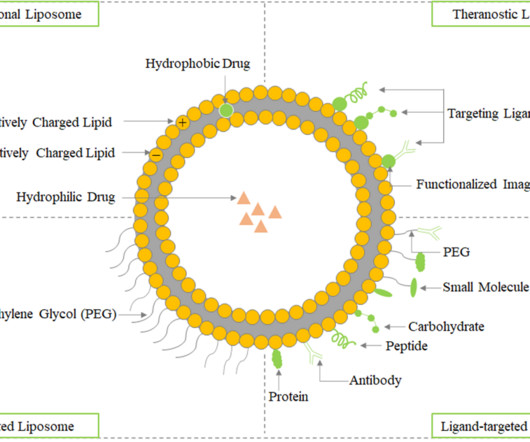
Roots Analysis
AUGUST 8, 2023
The hydrophobic portion contains two fatty acid chains with 10-24 carbon atoms, while the hydrophilic part primarily consists of phosphoric acid linked to a water-soluble molecule. Therapeutic Applications of Liposomes in Drug Delivery In comparison to existing formulations, liposomes offer greater therapeutic efficacy and safety.
Outsourcing Pharma
JANUARY 30, 2024
Two new offerings have been unveiled by drug delivery and solutions provider, Stevanto Group S.p.A.

Pharmaceutical Technology
SEPTEMBER 20, 2022
The information contained within the download document is intended for pharmaceutical manufacturers, wholesalers, retailers and distributors, pharmaceutical executives, medical representatives, business development managers, retail salesmen, sales managers, pharmacy executives, and any other individual involved in pharmaceutical marketing.

Pharmaceutical Technology
FEBRUARY 27, 2023
Antares Pharma overview Antares Pharma is a specialty pharmaceutical company engaged in the development, manufacturing, and commercialization of pharmaceutical products and technologies. Antares Pharma’s injectables are available in disposable multi-dose pen injectors and unit dose containers.

XTalks
MAY 30, 2023
The patch itself is made of biodegradable nanocellulose and contains no plastic additives. The healthcare industry has one of the heaviest environmental footprints, and manufacturers are increasingly faced with regulations to make more sustainable products,” said Mohammad H. Behfar, senior scientist at VTT, in the news release.
STAT News
NOVEMBER 3, 2022
The companies allege Moderna’s vaccine uses their technology for a drug-delivery system without permission. Citing Lilly announced earlier this year that the creation of its proposed manufacturing campus would create 300 permanent jobs once operational as well as 500 jobs during the construction phase.

XTalks
MAY 18, 2023
The wireless charger — the transmitter — contains a metallic coil that converts electrical energy into a magnetic field, which is then collected and reverted into electricity by the receiver inside the implanted device. Our wireless charging for titanium cans changes all of that.” billion in 2022.
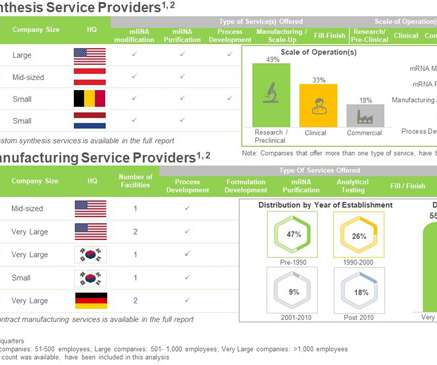
Roots Analysis
FEBRUARY 13, 2023
As a result, there is an evident increase in the demand for mRNA manufacturing capacity. The key applications of mRNAs are below: Contract Manufacturing in mRNA Synthesis AND Manufacturing Service Domain The synthesis and manufacturing process of mRNA-based therapeutics / vaccines is complex and associated with several challenges.

The Pharma Data
JANUARY 27, 2021
Construction of commercial drug manufacturing facility in Germany. Development of oral biosensor and contract development & manufacturing. Psychedelic transdermal and sublingual drug formulation development. Four clinical studies in neurological indications in 2021.

XTalks
JULY 28, 2020
Excipients can take many forms, such as bulking agents, binders, coatings and colourants, and they are chosen based on their ability to aid in drug delivery, administration and even in pill identification and prevention of counterfeiting. But just how inert are these excipients?

The Pharma Data
DECEMBER 20, 2020
With this acquisition we are expanding our product pipeline to include psychedelic therapeutics, incorporating elements of our IP around drug delivery technology in which we already have prototypes developed, which we believe will propel us towards clinical studies relatively quickly. Ahmad Doroudian, CEO of BetterLife.
Druggist
OCTOBER 21, 2020
Curanail Once Weekly 5% Fungal Nail Treatment Curanail Once Weekly Fungal Nail treatment contains 5% of amorolfine hydrochloride as an active ingredient. delivery Excilor – review of clinical effectiveness Sleven et al. Products which were found inactive in the experiment did not contain any organic acids.

The Pharma Data
NOVEMBER 2, 2020
“Psychedelic agents are a promising new class of therapeutic drug. We see a natural opportunity to apply XPhyto’s drug formulation expertise and Vektor’s proven drug delivery platforms to these emerging APIs,” said Hugh Rogers, CEO and Director of XPhyto. About XPhyto Therapeutics Corp.

Roots Analysis
JUNE 6, 2024
The continuous evolution of autoinjectors reflects a significant shift towards empowering patients to take control of their treatment, ensuring timely and accurate medication delivery for better health management. In January 2024, Rx Bandz signed a manufacturing agreement with Weiss-Aug for their MiniJect autoinjector.

Roots Analysis
JANUARY 17, 2024
They offer several advantages over traditional drug delivery systems ( such as vials and syringes ), including reduced chances of dosing errors, increased patient compliance and decreased risk of microbial contamination. However, this needle system does not contain a proper locking system, therefore, increased the chances of leakage.
Roots Analysis
FEBRUARY 18, 2022
Myeloid cells represent a group of cells containing granulocytes, monocytes, macrophages, mast cells and dendritic cells, which play a key role in innate immunity. These cells are found in abundance in the tumor microenvironment and inflamed areas. Myeloid Cells Targeting Therapeutics Market. Our Social Media Platform. Web: [link].

The Pharma Data
JANUARY 3, 2021
This press release contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. Except as required by law, TLC expressly disclaims any responsibility to update any forward-looking statement contained herein, whether as a result of new information, future events or otherwise.

The Pharma Data
DECEMBER 14, 2020
(“BetterLife” or the “Company”) (CSE: BETR / OTCQB: BETRF / FRA: NPAU), an emerging biotech focused on the development and commercialization of cutting edge treatments, today announced the value proposition of its acquisition of Transcend Biodynamics, LLC ’s (“Transcend”) manufacturing patent “Process of synthesizing 2-Bromo-LSD.”.

The Pharma Data
OCTOBER 20, 2020
” Mr. Bliss joins TLC following interim roles in cellular therapy, drug delivery and combination therapy where he led efforts to confirm research outcomes and partner the various technologies. This press release contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995.

The Pharma Data
JANUARY 25, 2021
Certain assets, including gold nanoparticle manufacturing equipment were transferred to Cardiff and others were sold. ” This announcement contains inside information for the purposes of Article 7 of Regulation (EU) 596/2014 (MAR). We are well positioned for a productive 2021.”

The Pharma Data
JANUARY 3, 2021
1 Good Manufacturing Practice. Nanoform’s capabilities span the small to large molecule development space and the company focuses on solving key issues in drug solubility and bioavailability and on enabling novel drug delivery applications. Edward Hæggström, CEO. edward.haeggstrom@nanoform.com. 358 29 370 0150.

The Pharma Data
NOVEMBER 4, 2020
He has 25 years’ experience including executive roles with major international European and Asian-based pharmaceutical companies and has more recently led a number of successful ventures in the pharmaceutical manufacturing, nutraceutical and other industry sectors. Thoresen is an important addition to XPhyto’s board of directors.

The Pharma Data
JANUARY 17, 2021
The Company will continue to leverage its scientific expertise and operations in Europe and North America for product development and optimization while it plans to add significant commercial experience in the fields of manufacturing, distribution, marketing and sales. About XPhyto Therapeutics Corp. XPhyto Therapeutics Corp.

The Pharma Data
DECEMBER 17, 2020
In addition, TLC is preparing for scale-up production of TLC590, as for complex lipid products, the manufacturing process and batch size used in pivotal clinical trials and New Drug Application submission must be the same as future commercial batches. Cautionary Note on Forward-Looking Statements. Source link.

The Pharma Data
DECEMBER 27, 2020
This press release contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. Except as required by law, TLC expressly disclaims any responsibility to update any forward-looking statement contained herein, whether as a result of new information, future events or otherwise.

The Pharma Data
NOVEMBER 9, 2020
On November 3, 2020, the Company announced a research agreement with a leading German university for the exclusive development of a proprietary biotechnology process for the industrial manufacture of psilocybin as a certified active pharmaceutical ingredient (“API”). About XPhyto Therapeutics Corp. XPhyto Therapeutics Corp.

The Pharma Data
NOVEMBER 4, 2020
(TSX: CTX) (OTC US: CRRTF) (“Crescita” or the “Company”), a growth-oriented, innovation-driven Canadian commercial dermatology company with in-house research & development (“R&D”) and manufacturing capabilities, today announced that it has entered into an exclusive agreement with Juyou-Biotechnology Co.

Delveinsight
JANUARY 22, 2021
The drug provides an alternative to the current replacement of ADAMTS13 using large volumes of fresh frozen plasma that contain variable amounts of ADAMTS13 and typically require two hours or more for the preparation and infusion.

FDA Law Blog
MARCH 12, 2024
Koblitz — After years of silence from FDA on whether certain patents could be listed in the Orange Book, some manufacturers of drug and device combination products have had a rude awakening lately.

The Pharma Data
JANUARY 15, 2021
and Genmab A/S entered into a worldwide agreement, which granted Janssen an exclusive license to develop, manufacture and commercialize daratumumab. Darzalex Faspro® is co-formulated with recombinant human hyaluronidase PH20 (rHuPH20), Halozyme’s ENHANZE® drug delivery technology. Janssen Biotech, Inc.

Pharmaceutical Technology
FEBRUARY 3, 2023
While the industry is seeing advances in alternative drug delivery systems, oral solid doses, such as pills, capsules and soft gels, remain at the forefront of the industry. Tablet manufacturing, which is the most common OSD form, takes an API dry powder ingredient and compresses it to form tablets, which can be coated or uncoated.

Pharmaceutical Technology
APRIL 16, 2023
But with the advent of injection site absorption enhancers, drug delivery device innovations, as well as the recent primary container options, the practical subcutaneous self-administration of large-volume parenterals is becoming possible. For them, intravenous infusion in a hospital setting is the only option. autoinjector.

XTalks
AUGUST 30, 2022
Big pharmaceutical companies require a system of hierarchy to accurately adhere to the legal and regulatory requirements for drug delivery, drug approval, supply chain and manufacturing processes. Although these leadership styles are of very different natures, they also contain some commonalities.
XTalks
JANUARY 23, 2025
PBMs serve as intermediaries between insurers, pharmacies and drug manufacturers, negotiating discounts and managing drug formularies. While PBMs were originally intended to lower costs and streamline drug delivery, critics argue that their practices often lead to inflated prices and restricted access for consumers.
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?


Let's personalize your content