The Burgeoning Hemp Beverage Market + Why is Tara Flour Unsafe? – Xtalks Food Podcast Ep. 163
XTalks
JUNE 13, 2024
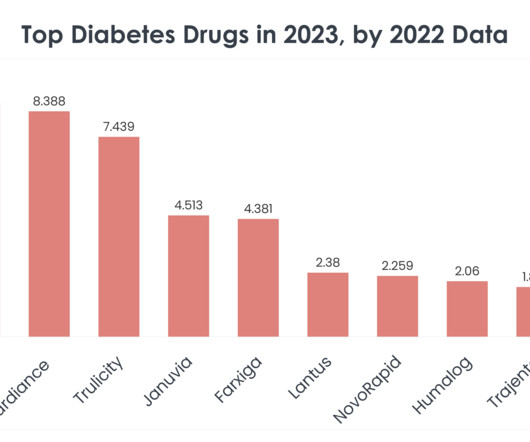
The market for hemp beverages has been growing rapidly in recent years. The passing of the 2018 Farm Bill, which legalized hemp production, boosted the market, allowing companies to explore hemp as a primary ingredient. This ruling, dated May 15, 2024, is only the 15th such declaration by the agency since 2010.














































Let's personalize your content