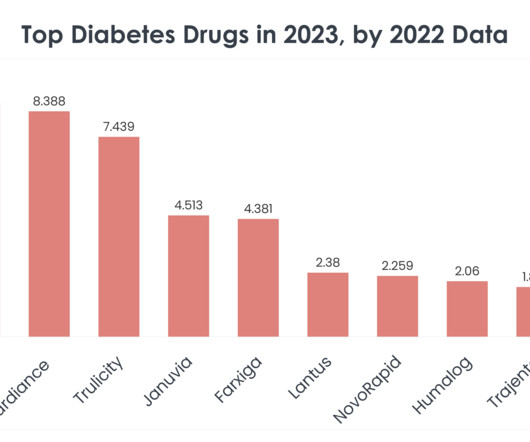
Top 15 Diabetes Drugs in 2023 by 2022 Sales Statistics
XTalks
FEBRUARY 8, 2024
The top 15 diabetes drugs in 2023, according to 2022 sales data, reflect the current state of diabetes management and hint at the evolving needs and trends within this critical area of healthcare. Read on to learn more about the top 15 diabetes drugs in 2023, based on 2022 sales statistics. mL subcutaneous solution (2 mg/1.5






























Let's personalize your content