Genotoxicity Testing: Unlocking the Future Safety Assessment Opportunities
Roots Analysis
MARCH 1, 2023
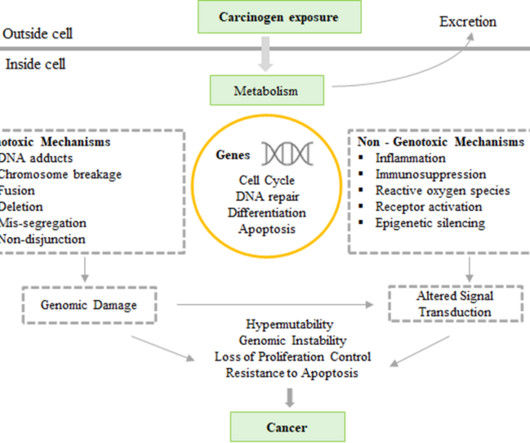
Genotoxicity testing refers to the evaluation of detrimental effects of chemical or physical agents on the genetic processes and related hereditary material of living cells. Mechanism of Genotoxicity / Mutagenicity The interaction of genotoxins / mutagens with the structure of DNA causes damage to the genetic material.














Let's personalize your content