Lab to jab in 100 days: manufacturing flexibility for future rapid responses
Pharmaceutical Technology
NOVEMBER 22, 2022
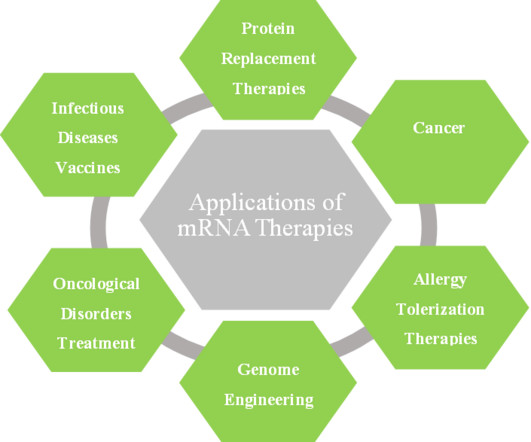
According to CEPI: “Achieving the 100 Days Mission would give the world a fighting chance of containing a future outbreak before it spreads to become a global pandemic.”. Messenger RNA vaccines contain nucleic acids that code for a specific protein, or target antigen, related to a virus or disease. So, where do we go from here?


















Let's personalize your content