FDA Warns About Compounded Versions of Ozempic and Wegovy
XTalks
JUNE 5, 2023
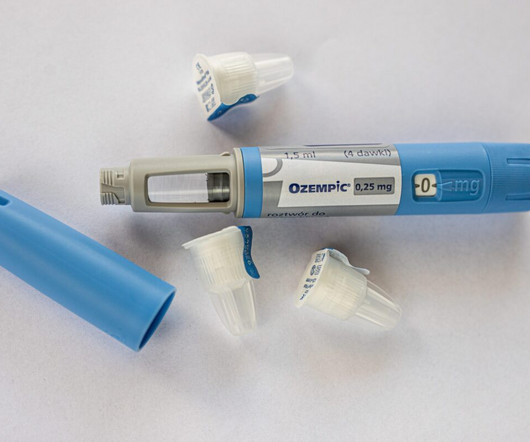
Ozempic and Wegovy, which contain the active ingredient semaglutide, were approved by the US Food and Drug Administration (FDA). GLP-1 stimulates insulin production, thus reducing blood glucose levels, and it interacts with the brain to suppress appetite and create a feeling of fullness. mg and 2.4 mg doses of Wegovy.











Let's personalize your content