UK MHRA publishes guidance on medicines containing valproate
Pharmaceutical Technology
OCTOBER 12, 2023
The UK Medicines and Healthcare products Regulatory Agency (MHRA) has published updated guidance on valproate-containing medicines.
This site uses cookies to improve your experience. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country, we will assume you are from the United States. Select your Cookie Settings or view our Privacy Policy and Terms of Use.
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.
Used for the proper function of the website
Used for monitoring website traffic and interactions
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.
Pharmaceutical Technology
OCTOBER 12, 2023
The UK Medicines and Healthcare products Regulatory Agency (MHRA) has published updated guidance on valproate-containing medicines.
Pharmaceutical Technology
NOVEMBER 30, 2022
A vitally important assay used across various stages of drug development and manufacturing, container closure integrity testing (CCIT) involves evaluating packaging systems to determine their ability to protect the stability and sterility of pharmaceutical products. The evolution of CCIT. Many test methods have thus been developed.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Pharmaceutical Technology
DECEMBER 6, 2022
Despite the current emphasis on biologic medicines, small molecule drugs still dominate the pharmaceutical manufacturing world. Cross-contamination of standard products with highly potent ones is another key concern. Many companies are now prioritising investments in containment systems, GlobalData research shows.

pharmaphorum
SEPTEMBER 2, 2020
The UK’s medicines regulator has published long-awaited guidance on regulation of medicines and medical devices as the UK approaches the end of its Brexit transition period at the end of the year. The concern is that UK patients will face delays to receiving the latest medicines because of the changes to the regulatory system.

Druggist
DECEMBER 17, 2020
Without a doubt, codeine-containing products are one of the most commonly sold medicines in the UK. Most codeine products are combination products of codeine and another drug. Restrictions on the supply of codeine-containing products. Codeine over the counter: UK list of products.

Druggist
NOVEMBER 10, 2020
Today I will list Calpol alternative products, including other brands and forms of liquid paracetamol and alternative drugs. Although the intention is to find Calpol alternative products for toddlers and children, I included a few products, which have a minimum age restriction of 12 years and above. Calpol alternative products.

Druggist
MARCH 11, 2021
This side effect is sometimes associated with the whole class of medicines rather than a particular drug, making dry mouth management even more critical, as there may be little room for changing the medication. Saliva production is primarily driven by mechanical and gustatory (taste) stimulation (Dodds et al., Best dry mouth lozenges.

Pharmaceutical Technology
APRIL 4, 2023
Abacus Medicine Pharma Services (AMPS) and Laboratoires CTRS, marketing authorization holder of Orphacol®, are proud to announce a strategic partnership that ensures continuous stock of Orphacol® in Spain. Orphacol® is a pharmaceutical product containing cholic acid, a substance found in the bile, which is used to digest fats.

Pharma in Brief
APRIL 25, 2021
These amendments permit patents claiming different forms of a medicinal ingredient (e.g., As previously reported , the Proposed FDR Amendments were intended to clarify the regulatory requirements under the Abbreviated New Drug Submission (“ ANDS ”) pathway for generic drug products that contain different forms (e.g.,

XTalks
FEBRUARY 21, 2022
BioNTech announced its new vaccine manufacturing approach in Africa where the company will ship modular factories, called the BioNTainer, to the continent to enable domestic production of the vaccines to help increase supplies. Related: BioNTech to Build First mRNA Vaccine Production Plant in Africa. Photo source: BioNTech.

Druggist
NOVEMBER 6, 2020
Chesty cough, also known as a productive cough, is characterised by overproduction of mucus. Patients commonly request over the counter medicines for a chesty cough. In today’s post, I will seek to find the best medicine for chesty cough by looking at over the counter and pharmacy-only products available in the UK.

Druggist
AUGUST 14, 2020
Gaviscon range of products is probably the most known brand of antacids in the UK. There are, however, several alternative products and drugs which can be used to help with symptoms of heartburn and indigestion. Today I will review 16 best Gaviscon alternative products and medicines.

Pharmaceutical Technology
SEPTEMBER 5, 2022
Pharmaceutical wholesalers act as intermediaries between pharmaceutical manufacturers and retailers and facilitate the delivery of the right medicines in a timely, efficient, and secure manner. Wholesale distributors are responsible for guaranteeing product quality and preventing the influx of counterfeited drugs. GDP and GMP advisory.

Camargo
NOVEMBER 11, 2020
Unpacking the (Black) Box: Antares Licenses Urology Product with Boxed Warning. When the FDA requires a product’s labeling to include a boxed warning (also called a “black box warning” because the text is surrounded by black border), the potential market value of the drug often drops severely. In October, the U.S.

pharmaphorum
NOVEMBER 16, 2020
The EMA’s human medicines committee has recommended approval of a fixed-dose combination of Roche’s breast cancer drugs Herceptin and Perjeta – part of the company’s defense against Herceptin biosimilars. According to the committee, the combination offers “less invasive and faster administration as a single product” to patients.

Drug Patent Watch
JANUARY 14, 2025
The Thyroid Association’s Concerns The American Thyroid Association (ATA) had raised concerns about the FDA’s methods for determining bioequivalence between levothyroxine products[2]. They recommended avoiding switching between levothyroxine products, leading to a preference for brand-name prescriptions.

Pharmaceutical Technology
SEPTEMBER 15, 2022
Packaging plays a vital role in maintaining the quality, safety, user-friendliness and marketability of drugs and other pharmaceutical products. The download contains detailed information on commercial pharmaceutical packaging contractors, and their product and service lines, alongside contact details to aid your hiring decision.
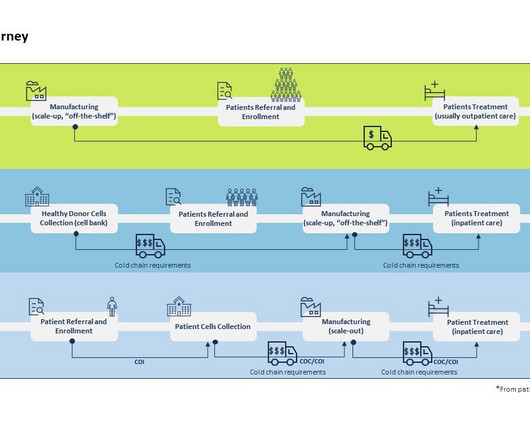
Pharmaceutical Technology
MAY 24, 2023
The ability to modify or introduce genetic material in human cells in such a precise and patient-centered manner clearly constitutes a breakthrough in personalized medicine. Allogeneic therapies begin with healthy donor samples to develop the eventual therapeutic product which can be administered to multiple patients.

Camargo
SEPTEMBER 14, 2021
As regulatory requirements become increasingly harmonized across the globe, the development and marketing of pharmaceutical products worldwide are also becoming more streamlined. INDs must contain full study reports of nonclinical and clinical (if available) studies.
Pharmaceutical Technology
AUGUST 15, 2022
The UK Medicines and Healthcare products Regulatory Agency (MHRA) has granted conditional authorisation for Moderna ’s Covid-19 booster vaccine, mRNA-1273.214 (Spikevax Bivalent Original/Omicron), for use in adults aged 18 years and above.

pharmaphorum
JANUARY 20, 2022
According to the company, the defendants reportedly sold 85,247 bottles of medicine with counterfeit Gilead labelling to pharmacies over a two-year period, using falsified supply chain documentation to conceal their origin. This works to ensure the safety and efficacy of the drugs received by patients.

Pharmaceutical Technology
JUNE 8, 2023
Arexvy contains a combination of recombinant glycoprotein F stabilised in the prefusion conformation (RSVPreF3) and GSK’s AS01E adjuvant. The EC approval follows a positive opinion from the European Medicines Agency’s committee on medicinal products for human use (CHMP) in April 2023.
Pharmaceutical Technology
JUNE 21, 2023
In the past year, Hemgenix has been granted approval by the US Food and Drug Administration (FDA) and has received conditional marketing authorisation from the European Commission (EC) and the UK’s Medicines and Healthcare products Regulatory Agency (MHRA). This followed positive results from CSL’s Phase III HOPE-B trial (NCT03569891).

Pharmaceutical Technology
SEPTEMBER 20, 2022
The production of commercial dose non-sterile products such as tablets, capsules, ointments, creams, and powders is rising due to their growing global demand, attributed to increasing demand for anti-ageing products, hereditary factors, genetic mutations, exposure to harmful radiation, and rising geriatric population.

Pharmaceutical Technology
DECEMBER 4, 2022
It is all about what you can get at the lowest cost,” says Dr. Raymond Cross, director of the inflammatory bowel disease program at University of Maryland’s School of Medicine, Baltimore. Product variation creates patient uncertainty. However, there tend to be some differences between the products.

STAT News
SEPTEMBER 29, 2022
” The proposed label rule aims, in part, to address a question as old as medicine: What does it mean for a food to be healthy? It would update the “healthy” label guidelines from 1994 to match up-to-date nutrition research — a notoriously messy and heavily debated field. Read the rest…

Druggist
JANUARY 15, 2021
Paracetamol, one of the most prescribed drugs in the UK , and probably the most popular over the counter medicine has been recently subject to shortages due to increased demand in recent months. Firstly, I will list some common combination of drugs, which contain paracetamol, which may be used if standard paracetamol tablets or capsules.
Pharmaceutical Technology
FEBRUARY 8, 2023
The recent town hall, titled “Clinical Development of Gene Therapy Products for Rare Diseases”, was meant to further that dialogue with those in pharmaceutical industry developing new gene therapies for underserved patient groups.

Pfizer
JULY 19, 2022
Pfizer and BioNTech Complete Submission to European Medicines Agency for Omicron BA.1 Pfizer and BioNTech Complete Submission to European Medicines Agency for Omicron BA.1 is immunocompromised or are on a medicine that affects the immune system. The information contained in this release is as of July 19, 2022.
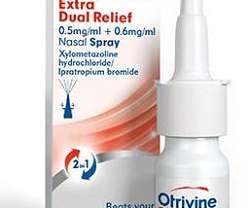
Druggist
AUGUST 3, 2020
Otrivine Extra Dual Relief is a pharmacy only medication and the only over the counter product containing ipratropium bromide for the management of congestion and runny nose caused by colds. P medicines can only be purchased from a pharmacy, including an online pharmacy with a presence of a pharmacist.

Druggist
MARCH 23, 2021
Ibuprofen prescribing decreased significantly in recent years due to the NHS strategy to minimise issuing of medicines that are available over the counter. Nurofen comes in the form of tablets and liquid capsules, marketed for the management of different conditions, despite no difference in the active ingredient in each product.

Pharmaceutical Technology
SEPTEMBER 8, 2022
The expert services rendered by them help in improving patient outcomes, minimising costs of the healthcare system, improving medicinal adherence and persistency and saving clinicians’ time. The list includes companies that offer various products and services, including but not limited to: · Special handling services.

Pfizer
SEPTEMBER 28, 2022
Pfizer and BioNTech Complete Submission to European Medicines Agency for Omicron BA.4/BA.5-Adapted Pfizer and BioNTech Complete Submission to European Medicines Agency for Omicron BA.4/BA.5-Adapted 5-Adapted Bivalent Vaccine Booster in Children 5 Through 11 Years of Age. Tue, 09/27/2022 - 16:15. Wednesday, September 28, 2022 - 11:45am.

Pharmaceutical Technology
SEPTEMBER 8, 2022
Pharmaceutical compounding companies are expanding geographically and launching innovative products according to customer requirements to get a better foothold in the market. Lab batch production. Pharmaceutical compounding may fulfil the specific requirements of patients, unlike mass-produced medicines. Excipients. Formulation.

pharmaphorum
MARCH 26, 2021
Medicinal products are mentioned only in passing. This applies equally to medicinal products and medical devices as to any other type of goods. Even where products do originate in the UK or EU, thus qualifying for tax and duty-free status, this does not mean they can cross the border without friction.
Roots Analysis
AUGUST 29, 2024
In the realm of modern medicine, nuclear medicine has emerged as a promising and advanced tool that can be used for the diagnosis and treatment of diseases. As such there are several factors which will continue to drive radiopharmaceutical production market. This device is mostly used in the production of Technetium-99m.

Druggist
SEPTEMBER 7, 2020
All of the non-prescription sleeping pills available in the UK contain sedative antihistamines. Over the counter sleeping pills UK: active ingredients All products available over the counter ‘for sleeping’ contain sedative antihistamines as the main active ingredient. Many factors can cause insomnia.

Druggist
JANUARY 9, 2021
Where possible, I will review the evidence to answer what the best over the counter migraine medicine is. Over the counter codeine-based medicines. Smaller packs of paracetamol (contain 16 tablets or capsules) are available in all supermarkets and pharmacies for patient self-selection. Over the counter sumatriptan for migraine.

Camargo
DECEMBER 13, 2021
The development of biological products (or biologics) represents a major advancement in modern medicine, enabling the treatment of patients with many illnesses where no other therapeutics were previously available. The regulations regarding BLAs for therapeutic biological products are included in 21 CFR parts 600 , 601 , and 610.

Pharmaceutical Technology
SEPTEMBER 14, 2022
Peptide API products have become popular due to their proven pharmacological value and favourable safety profiles. The increasing demand for API peptide products is in turn increasing the demand for peptide API manufacturers who also offer contract marketing services.

Pharmaceutical Technology
MAY 25, 2023
If the company chooses not to pursue these in some countries, the rights to the product there will be returned to the Swiss company in a pre-defined reasonable timeframe. With a network of more than 134 local pharmaceutical companies, Adalvo supplies more than 110 different medicines in over 100 countries across the world.
pharmaphorum
JANUARY 30, 2023
The supply issues appear to be broadly impacting a number of different antibiotics, including amoxicillin, penicillin, and ceftolozane, among other products. For its part, the US Food and Drug Administration (FDA) posted its drug shortage list , which contains various formulations of amoxicillin that are limited in supply.

Scienmag
FEBRUARY 7, 2021
Disconnects between the front labels and ingredient lists of packaging containing fruits and vegetables make it more difficult for parents to understand what kind of food they are buying for their children, according to the Journal of Nutrition Education Credit: Journal of Nutrition Education and Behavior Philadelphia, February 8, 2021 – Early (..)
Druggist
APRIL 16, 2021
Throat numbing lozenges contain a local anaesthetic which numbs the area and hence help with pain and irritation. This post list all sore throat lozenges containing a local anaesthetic available in the UK. Sore throat lozenges, which contain a local anaesthetic as an active ingredient, are classified as pharmacy-only products (P).
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?


Let's personalize your content