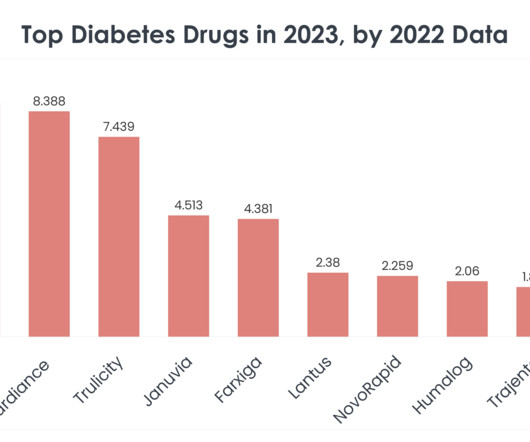
Top 15 Diabetes Drugs in 2023 by 2022 Sales Statistics
XTalks
FEBRUARY 8, 2024
Understanding the market dynamics of diabetes treatments becomes crucial for professionals across these industries. Ozempic (Semaglutide) Ozempic sales in 2022: $8.713 billion Company/Developer: Novo Nordisk Date of first FDA approval: December 5, 2017 Indications Ozempic is FDA-approved for: Type 2 diabetes Price of Ozempic: $1,029 for 1.5















Let's personalize your content