Indian pharma manufacturing: rising investment in Andhra Pradesh
Pharmaceutical Technology
APRIL 4, 2023
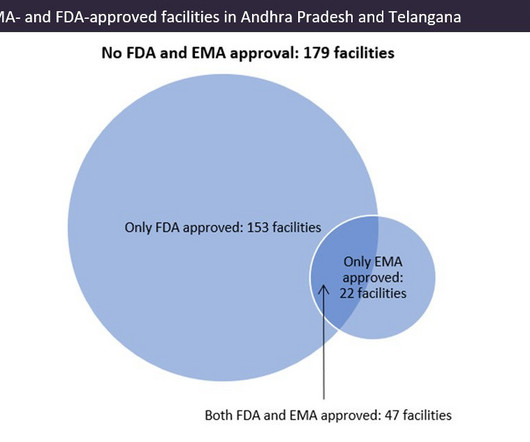
Indian pharma manufacturing continues to be the backbone of drug supplies worldwide, and GlobalData analysis suggests US overreliance on the country for generic drug supply. Source: GlobalData, Pharma Intelligence Center Drug Database (Accessed March 20, 2023). ©GlobalData.














Let's personalize your content