Why small particles matter: Manufacturing and micro-material contamination
Pharmaceutical Technology
MARCH 6, 2024
To protect patient safety and reduce recall risks, drug manufacturers must reduce particle contamination sources wherever possible.
This site uses cookies to improve your experience. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country, we will assume you are from the United States. Select your Cookie Settings or view our Privacy Policy and Terms of Use.
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.
Used for the proper function of the website
Used for monitoring website traffic and interactions
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.

Pharmaceutical Technology
MARCH 6, 2024
To protect patient safety and reduce recall risks, drug manufacturers must reduce particle contamination sources wherever possible.

Bio Pharma Dive
APRIL 22, 2021
A recent nine-day inspection by agency officials found numerous breaches of manufacturing standards and inadequate training for factory employees.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Drug Patent Watch
JANUARY 22, 2025
The Unseen Heroes of Generic Drug Manufacturing: Good Manufacturing Practice (GMP) As we navigate the complex world of generic drug manufacturing, it's easy to overlook the unsung heroes that ensure the quality and safety of these life-saving medications. But what exactly does GMP entail?

Pharmaceutical Technology
FEBRUARY 3, 2023
Additionally, OSD manufacturing approaches are well developed, with processes that ensure a repeatable distribution of ingredients, uniformity in dissolution, and bioavailability, which verify that the drug product is safe and effective. Compared to other dosage forms, tablets are simpler to manufacture, package, and transport.

Bio Pharma Dive
APRIL 15, 2024
Because live cell and tissue products are extremely sensitive to contamination from microorganisms, viral particles or other airborne impurities, research and manufacturing involving these materials needs to be conducted in a cleanroom environment.

Fierce Pharma
SEPTEMBER 25, 2023
GSK manufacturing review prompts Scynexis to pull Brexafemme, halt trials over contamination concerns esagonowsky Mon, 09/25/2023 - 12:26

XTalks
JULY 16, 2021
Johnson & Johnson is recalling sunscreens from two of its popular brands over concerns of carcinogenic benzene contamination. Therefore, levels should be monitored at different stages of production to avoid harmful benzene contamination. Growing Contamination Issues. Despite this, contamination issues still persist.

Fierce Pharma
SEPTEMBER 19, 2024
From truckloads of torn documents to avian incursions in the production plant, Granules India’s recent manufacturing reprimand from the FDA is alarming no matter which way you look at it. The FDA’s report cites six observations around poor quality control procedures, subpar cleaning, cross-contamination risks and more. 26 to Sept.

Fierce Pharma
JUNE 6, 2024
When the FDA comes knocking at your drug manufacturing facility, it’s best to play along. |
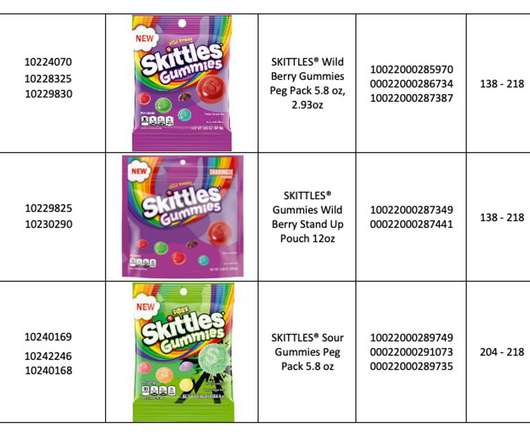
XTalks
MAY 19, 2022
According to the company, products were manufactured by a third party and subsequently distributed across North America. Since metal contamination is a common issue in food production, resulting in expensive recalls and lawsuits, the FDA has implemented metal detection standards to ensure the same principles are followed for all companies.
STAT News
AUGUST 22, 2022
After a decade of manufacturing problems, a U.S. federal court ordered a unit of Wockhardt, one of the largest makers of generic drugs, to refrain from making allegedly adulterated medicines at a facility in Illinois. However, the U.S. Continue to STAT+ to read the full story…

Pharmaceutical Technology
AUGUST 17, 2022
NTI have tackled this problem through the introduction of its NEBULAE® SRS system , which filters and recycles the air in surgical theatres, removing potentially harmful contaminates. has been manufacturing, designing, and innovating since 1984. Pioneers in minimally invasive surgical technology, Northgate Technologies Inc.

pharmaphorum
MARCH 22, 2022
In this article, Ben Hargreaves asks whether there is particular potential for the technologies to revolutionise staff training and reduce costs in pharma manufacturing. The importance of manufacturing. the streamlining of pharma manufacturing in the 21st century. Taking manufacturing to the next level.

Pharmaceutical Technology
DECEMBER 8, 2022
This process is used to manufacture pharmaceutical products with low molecular weights in large batches within a short timescale. While this is exciting news for the pharmaceutical industry, chemical synthesis presents a number of challenges when it comes to running a manufacturing plant. Chemical synthesis solutions.

Pharmaceutical Technology
OCTOBER 26, 2022
Nano-based delivery systems are on the rise, as they enable manufacturers to deliver therapeutic agents to specific targeted tissue in a more controlled manner. Regulations and guidelines for nanopharmaceuticals are still relatively in their infancy, including for cleaning processes and the prevention of cross-contamination.

Drug Patent Watch
FEBRUARY 19, 2025
From inspecting manufacturing facilities to testing finished products, QA teams work tirelessly to guarantee that every pill, tablet, or capsule that reaches the market is safe and effective. But today, I want to shine a spotlight on the often-overlooked heroes of the generic drug industry: Quality Assurance (QA) professionals.

Roots Analysis
JANUARY 27, 2025
It is worth mentioning that the past few decades have witnessed several advances in this domain, particularly in the cell therapy manufacturing process. Steps Involved in Cell Therapy Manufacturing Process Manufacturing biologics and cell therapies is considerably complex when compared to small molecule drugs.

XTalks
APRIL 10, 2025
Its platform delivers ingredient solutions for both chefs and food manufacturers, all while diverting tons of waste. In 2025, the company faced US import tariffs on goods from China where it manufactures its products prompting adjustments to its supply chain to continue sourcing key regional ingredients.

BioPharma Reporter
OCTOBER 6, 2021
A probe has identified 'human errorâ at the Spanish contract development and manufacturing organization, Rovi, as the reason for the presence of metal contaminants in Moderna COVID-19 vaccine doses, leading to Japanese authorities withdrawing 1.6 million doses of the shot in August.

STAT News
APRIL 3, 2023
Your tips and insights help the world go around… Global Pharma Healthcare, whose eye drops have been linked to dozens of serious reactions, failed to follow numerous procedures to ensure its products did not become contaminated , according to a U.S. Food and Drug Administration inspection report obtained by STAT.

Fierce Pharma
APRIL 10, 2024
FDA chided Kilitch Healthcare India for “poor practices” tied to written procedures around microbial contamination, shoddy lab records, behaviors that could have caused contamination and quality control lapses. . | In a four-observation warning letter issued this week, the U.S.

Pharmaceutical Technology
JANUARY 6, 2023
The preparation of a semi-solid dosage form – from formulation and development, to scale-up, commercial manufacturing, and packaging – should ideally take place under one roof in a contiguous, end-to-end workflow to avoid unnecessary equipment and process changes. Optimising development and scale-up. Bioburden and microbial testing.

BioSpace
APRIL 29, 2021
Battered by vaccine manufacturing mishaps that led to the contamination of 15 million doses of the Johnson & Johnson vaccine, Emergent BioSolutions is shaking up its leadership to ensure such errors do not occur again.

Medical Xpress
JANUARY 4, 2023
The researchers also determined the relative contribution of various sources of antibiotic contamination in waterways, such as hospitals, municipals, livestock, and pharmaceutical manufacturing.

STAT News
JANUARY 19, 2023
In a stunning rebuke, the Food and Drug Administration accused a drugmaker of a “cascade of failures” for a litany of quality-control problems at a manufacturing plant, the latest instance in which the regulator has castigated an Indian pharmaceutical company for such lapses. Continue to STAT+ to read the full story…

STAT News
MARCH 31, 2023
Global Pharma Healthcare, whose eye drops have been linked to dozens of serious reactions, failed to follow numerous procedures to ensure its products did not become contaminated, according to a Food and Drug Administration inspection report obtained by STAT.

STAT News
JANUARY 10, 2023
Food and Drug Administration for a host of serious manufacturing violations at a key plant in India, the latest instance in which the company was tagged by the regulator for quality-control problems.

Medical Xpress
FEBRUARY 16, 2023
Like any other manufactured food product, chocolate can be contaminated if key ingredients or processes break down.

XTalks
AUGUST 12, 2024
Lead, a toxic metal, can enter the food supply through environmental contamination during the growing, raising or processing of foods. FDA’s Regulatory Framework on Lead in Food Lead contamination in food can arise from various environmental sources, including past usage of lead in paint, gasoline and plumbing materials.

Medical Xpress
APRIL 17, 2023
An analysis of global usage patterns of a common hypertension drug following a major recall demonstrates the worldwide impact of contamination at a single manufacturing facility.

STAT News
AUGUST 15, 2022
communities. Although researchers have known about the impact of these chemicals for years, there has been little guidance available for clinicians.
XTalks
MARCH 25, 2022
Pfizer places the utmost emphasis on patient safety and product quality at every step in the manufacturing and supply chain process,” the company stated in a news release. Last summer, Pfizer recalled a dozen lots of its smoking cessation drug Chantix due to nitrosamine contaminants.

XTalks
DECEMBER 14, 2020
AvKARE, a generics pharmaceutical manufacturer based in Pulaski, Tennessee, has issued a recall on one lot each of its sildenafil 100 mg tablets and trazodone 100 mg tablets due to a packaging “mix-up” in which both drugs were filled into the same bottle. According to AvKARE, the error occurred at a third-party bottle filling facility.
XTalks
NOVEMBER 23, 2023
Salmonella Outbreak from Poultry — The US A widespread Salmonella outbreak linked to contaminated poultry affected thousands across multiple states this year. It also encouraged a citizen petition to make it illegal to sell poultry contaminated with any one of 31 strains of Salmonella. Coli Daycare Outbreak — Canada In September, an E.

XTalks
JULY 30, 2024
The US Department of Agriculture’s (USDA) Food Safety and Inspection Service (FSIS) declared Salmonella an adulterant in raw breaded stuffed chicken products when contamination levels exceed one colony-forming unit (CFU) per gram. This approach emphasizes stringent testing and preventive measures at earlier stages of the supply chain.

Pharmaceutical Technology
MARCH 23, 2023
When freeze drying sterile pharmaceuticals, mass spectrometry helps manufacturers protect valuable batches from silicone oil contamination – a possible risk of the lyophilisation process. Preventing silicone oil contamination Silicone oil is a common component of lyophilisation systems.

Pharmaceutical Technology
DECEMBER 20, 2022
Maintaining sterility is critical in the final steps of parenteral drug processing, ensuring doses are free from microbial contamination that could affect patient safety. With operator intervention being a primary source of contamination, finding ways to reduce human contact has been an ongoing challenge and goal in aseptic filling.

pharmaphorum
FEBRUARY 15, 2022
It found that the highest contamination levels were in in sub-Saharan Africa, south Asia, and South America, typically in areas of low- to middle-income countries that have poor wastewater management, rubbish dumping along river banks, and pharmaceutical manufacturing plants.

XTalks
SEPTEMBER 17, 2020
Sprouts can carry a risk of foodborne illness if they are contaminated, and unlike other fresh produce, the warm, moist conditions required to grow sprouts are ideal for the rapid growth of bacteria, including Salmonella , Listeria , and E. No confirmed illnesses related to Fortune Food’s products have been reported to the FDA.

Pharmaceutical Technology
NOVEMBER 17, 2022
According to GlobalData analysis, specialist small molecule API capabilities such as containment or controlled substances are driving large contract manufacturing organisations (CMOs) to acquire facilities.

Fierce Pharma
FEBRUARY 20, 2024
It’s been 15 years since Genzyme began rationing the Fabry disease treatment Fabrazyme after a shortage caused by contamination at a manufacturing site, marking the start of many years of litigatio | Sanofi's Genzyme was accused of causing injuries after contaminated doses of its Fabry disease drug led to a shortage, during which the company rationed (..)

Pharmaceutical Technology
MAY 2, 2023
The pharmaceutical industry is constantly striving for better, cleaner, safer manufacturing, especially when it comes to injectable medicines. Datwyler is a leading provider of parenteral packaging components and is helping the industry put quality first through the invention of the FirstLine® manufacturing standard.

XTalks
MARCH 10, 2022
Traditional food safety testing methods used in manufacturing facilities are inconsistent, time consuming and often require trained technicians. So, if a food manufacturer identifies a positive Salmonella result in its facilities, the impact of the positive result differs greatly depending on whether it is pathogenic or not.

XTalks
AUGUST 12, 2020
Thomson International, a company based out of Bakersfield, California, has recalled red, yellow, white and sweet yellow onions shipped from May 1, 2020 until now due to potential Salmonella contamination. of Bakersfield, California, USA, or any products made with these onions. More about Salmonella.
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?


Let's personalize your content