The rise of GLP-1 drugs: Transforming weight loss treatment
Bio Pharma Dive
JUNE 23, 2025
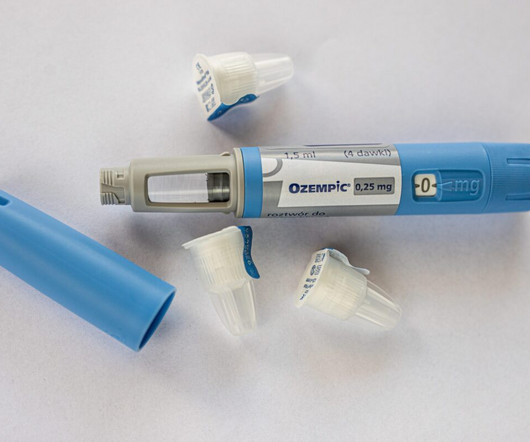
Originally developed in the 1970s to treat diabetes, these drugs—such as Ozempic, Wegovy, and Mounjaro—have become headline-makers for their ability to induce significant weight loss. Understanding GLP-1 drugs GLP-1, or glucagon-like peptide-1 receptor modulators, mimic natural hormones that regulate insulin and appetite.
















































Let's personalize your content