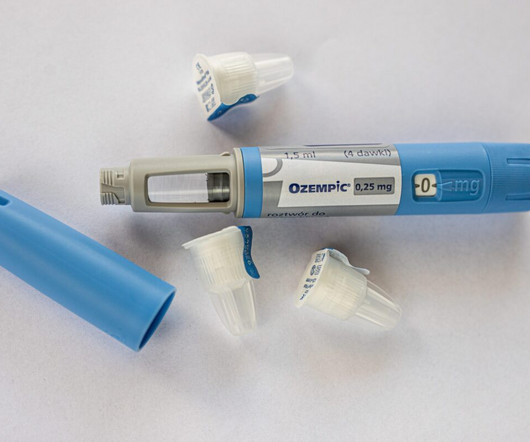
FDA Warns About Compounded Versions of Ozempic and Wegovy
XTalks
JUNE 5, 2023
Semaglutide acts as a glucagon-like peptide-1 (GLP-1) receptor agonist, mimicking the GLP-1 hormone released in the gastrointestinal tract after eating. Patients and healthcare professionals should understand that certain semaglutide products may not contain the same active ingredient as FDA-approved semaglutide.









Let's personalize your content