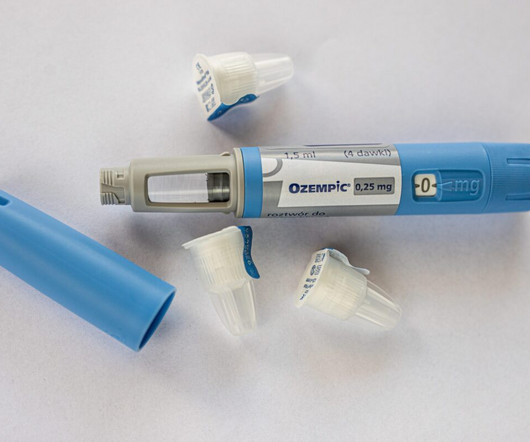
FDA Warns About Compounded Versions of Ozempic and Wegovy
XTalks
JUNE 5, 2023
During a drug shortage, compounders might be allowed to prepare a compounded version of the drug provided they meet specific requirements of the Federal Food, Drug, and Cosmetic (FD&C) Act. Novo Nordisk, the pharmaceutical manufacturer of Ozempic and Wegovy, is actively addressing these supply challenges.




















Let's personalize your content