Almirall and Microsoft partner for dermatological drug development
Pharmaceutical Technology
FEBRUARY 1, 2024
Almirall has entered a strategic collaboration with Microsoft to steer innovation and digital transformation in dermatology.
This site uses cookies to improve your experience. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country, we will assume you are from the United States. Select your Cookie Settings or view our Privacy Policy and Terms of Use.
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.
Used for the proper function of the website
Used for monitoring website traffic and interactions
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.

Pharmaceutical Technology
FEBRUARY 1, 2024
Almirall has entered a strategic collaboration with Microsoft to steer innovation and digital transformation in dermatology.

Pharmaceutical Technology
NOVEMBER 15, 2023
Almirall has entered into a collaboration with Absci for the development and commercialisation of new treatments for dermatological ailments.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

XTalks
NOVEMBER 7, 2024
Galderma’s doxycycline drug Oracea (doxycycline 40 mg capsules) is the current standard-of-care treatment for rosacea. Maraoui said the company’s dermatology-focused sales force is “now preparing for a successful launch and to establish Emrosi as a new standard of care in the treatment of rosacea.”

Pharmaceutical Technology
MAY 30, 2023
According to GlobalData’s Looking Ahead to 2023 – the Future of Pharma report, five drugs set for approval in 2023 are projected to attain blockbuster status or near-blockbuster status by 2028 with US company dominance. Genmab AS’s epcoritamab presents as the top European drug launch and only oncological therapy.

XTalks
OCTOBER 24, 2024
Meanwhile, Innovent Biologics recently saw its New Drug Application (NDA) for picankibart, an IL-23p19 inhibitor, accepted by the Chinese National Medical Products Administration (NMPA). This drug may significantly improve psoriasis care by offering effective long-term control with fewer side effects.

XTalks
OCTOBER 25, 2024
This collaboration aims to support patients with chronic and complex conditions, particularly in oncology, rheumatology and dermatology. Nearly 3,000 healthcare providers will benefit, as Shields’ broad payer network and access to limited distribution drugs will offer patients more treatment options and cost-saving support.

Pharmaceutical Technology
MAY 28, 2024
J&J has made its second dermatology deal this month, adding Numab’s bispecific antibody NM26 to its growing atopic dermatitis drug portfolio.

pharmaphorum
MAY 9, 2023
4th Dermatology Drug Development Summit Europe Mike.Hammerton Tue, 05/09/2023 - 20:11 Bookmark this

Pharmaceutical Technology
SEPTEMBER 29, 2022
The latest takeover is anticipated to boost the presence of Torrent in the dermatology segment. Holding a presence in the cosmetic dermatology sector, Curatio’s portfolio comprises more than over 50 brands that are commercialised in India. Among these facilities, four are approved by the US Food and Drug Administration (FDA).

pharmaphorum
OCTOBER 9, 2023
Champion Innovation at the 7th Dermatology Drug Development Summit Mike.Hammerton Mon, 09/10/2023 - 14:39 Bookmark this

Pharmaceutical Technology
MAY 19, 2023
The US Food and Drug Administration (FDA) has approved AbbVie’s Rinvoq (upadacitinib) for patients with Crohn’s disease who do not respond to TNF blockers, a common immune suppressant treatment for the condition. The FDA nod means Rinvoq is now approved for seven indications in gastroenterology, rheumatology and dermatology.

Pharmaceutical Technology
JANUARY 19, 2023
Concert’s patent portfolio includes oral Janus kinases JAK1/2 inhibitor, deuruxolitinib, to treat Alopecia Areata, an autoimmune dermatological disease. Sun Pharma stated that it will follow Concert’s plan to seek approval from the US Food and Drug Administration (FDA) for its new drug application in the first half of the year.

Outsourcing Pharma
MAY 23, 2022
With Veeva Link for Key People, LEO Pharma is looking to deepen its efforts to engage with knowledgeable medical professionals in the dermatology community.

Pharmaceutical Technology
JULY 12, 2022
Health Canada has accepted to review Arcutis Biotherapeutics’ New Drug Submission (NDS) for roflumilast cream 0.3% (ARQ-151) to treat plaque psoriasis in adult and adolescent patients. A validated target in dermatology, PDE4 is an enzyme that induces overactive immune responses.

BioSpace
APRIL 21, 2021
On the back of the two positive Phase III trials, Ortho said it will submit a new drug application (NDA) to the U.S. Food and Drug Administration (FDA) in the second half of 2022.

Pharmaceutical Technology
FEBRUARY 28, 2023
According to GlobalData, Phase I drugs for Melanoma have a 76% phase transition success rate (PTSR) indication benchmark for progressing into Phase II. GlobalData’s report assesses how KFA-115’s drug-specific PTSR and Likelihood of Approval (LoA) scores compare to the indication benchmarks. Buy the report here.

pharmaphorum
OCTOBER 2, 2024
Industry’s Destination to Discover & Develop Best-In-Class Drugs for Immuno-Inflammatory Skin Disease
Pharmaceutical Technology
APRIL 19, 2023
The US Food and Drug Administration (FDA) has accepted Arcutis Biotherapeutics ’ new drug application (NDA) for roflumilast foam 0.3% The regulator has set 16 December 2023 as a prescription drug user fee act (PDUFA) target action date for the decision on the application.

BioTech 365
DECEMBER 3, 2020
DermTech Presents Updates in Precision Medicine at 2020 Dermatology Drug Development Summit DermTech Presents Updates in Precision Medicine at 2020 Dermatology Drug Development Summit LA JOLLA, Calif.–(BUSINESS –(BUSINESS WIRE)–DermTech, Inc.
BioPharma Reporter
NOVEMBER 21, 2023
A partnership combining an integrated drug creation platform with a company specializing in dermatological expertise will deliver life-changing medicines to patients.

BioTech 365
MARCH 15, 2021
Dermatology Drugs Market Research Report 2020 – Global Industry Analysis and Growth Forecast to 2030 – ResearchAndMarkets.com Dermatology Drugs Market Research Report 2020 – Global Industry Analysis and Growth Forecast to 2030 – ResearchAndMarkets.com DUBLIN–(BUSINESS WIRE)–The “Dermatology Drugs Market Research (..)

FDA Law Blog
NOVEMBER 10, 2024
Food and Drug Administration (FDA) plays a pivotal role in fostering the development of treatments for rare diseases through its Orphan Products Grants Program. who intend to evaluate a drug, biologic, medical device, or food for medical purposes that targets a rare disease in a clinical trial. By Sarah Wicks & James E.

BioTech 365
JULY 6, 2021
Biotechnology, Pharma and Biopharma News – Research – Science – Lifescience ://Biotech-Biopharma-Pharma: Leo taps X-Chem to discover anti-inflammatory dermatology drugs.Leo taps X-Chem to discover anti-inflammatory dermatology drugs ntaylor Tue, 07/06/2021 – 07:16 from FierceBiotech: Biotech [link].

XTalks
AUGUST 14, 2024
The US Food and Drug Administration (FDA) has approved Galderma’s monoclonal antibody Nemluvio (nemolizumab) for the treatment of adult patients with the chronic skin condition prurigo nodularis. Monoclonal antibody therapy has been found to have fewer side effects when compared to immunosuppressant drugs.

Fierce Pharma
OCTOBER 25, 2024
Key drugs from Johnson & Johnson and Eli Lilly stand to bolster their respective cases in competitive dermatology markets with new data readouts.

Fierce Pharma
NOVEMBER 4, 2024
Dermatology drugmaker Journey Medical has trekked its Dr. Reddy’s Laboratories-partnered rosacea med across the FDA finish line as Emrosi. |

BioTech 365
SEPTEMBER 21, 2021
Journal of Drugs and Dermatology Publishes Clinical Study on Topix Pharmaceuticals, Inc. New Vitamin C E + green Tea Polyphenols Serum Journal of Drugs and Dermatology Publishes Clinical Study on Topix Pharmaceuticals, Inc. New Vitamin C E + green Tea … Continue reading →

Pharmaceutical Technology
APRIL 19, 2023
The technology for the pilot project, which is cleared for use by Health Canada and the US Food and Drug Administration (FDA), is a cloud-based platform that facilitates patient access to a dermatologist. In Canada, access to dermatological care is extremely limited, with only 1.7

BioTech 365
APRIL 26, 2021
United States Generic Drugs Market Report 2020-2026: Focus on CNS, Cardiovascular, Dermatology, Genitourinary/Hormonal, Respiratory, Anti-infective & Oncology – ResearchAndMarkets.com United States Generic Drugs Market Report 2020-2026: Focus on CNS, Cardiovascular, Dermatology, Genitourinary/Hormonal, Respiratory, Anti-infective & (..)

pharmaphorum
OCTOBER 1, 2021
Already a major player in atopic dermatitis with Dupixent, Sanofi looked to expand its position in the category earlier this year when it bought Kymab and its lead drug KY1005 for the skin disorder for $1.1 Now, it says the drug has delivered “exciting” results in a proof-of-concept trial. billion upfront.
STAT News
DECEMBER 2, 2022
And a fragmented clinical trials industry has made it a prime target for private equity, which often consolidates markets by merging companies.

BioTech 365
AUGUST 3, 2021
Eurofins Strengthens Its Global Leadership Position in Cosmetics and Personal Care Products Testing and Clinical Services and Grows Its Position in Dermatology Drug Testing Eurofins Strengthens Its Global Leadership Position in Cosmetics and Personal Care Products Testing and Clinical Services … Continue reading →
Pharmaceutical Technology
APRIL 17, 2023
QPS is preparing the pre-investigational new drug applications to be submitted to the US Food and Drug Administration (FDA) and the EU’s European Medicines Agency (EMA) for the CannQuit and ReneCann products. French company Eurofins Scientific is currently developing and manufacturing both products.
pharmaphorum
SEPTEMBER 16, 2022
Atopic dermatitis (AD) is a highly prevalent chronic dermatological disease, with symptoms which include itching, dry skin, severe pain, and inflammation – all of which create considerable stress in the daily lives of patients and often negatively affect their day-to-day and long-term well-being.
XTalks
AUGUST 2, 2023
Verrica Pharmaceuticals is a dermatology therapeutics company developing medications for skin diseases that require medical interventions. Tune into this episode to learn more about YCANTH and its approval, as well as the changing landscape of dermatology, from Dr. Eichenfield.
pharmaphorum
MAY 25, 2022
VP-102 is a drug-device combination which takes the form of a formulation of cantharidin delivered topically via a single-use applicator, and is also being developed for other dermatology applications including common warts and genital warts. Sterling was classified by the FDA as voluntary action indicated (VAI) last November.

BioSpace
NOVEMBER 2, 2020
“With limited oral therapeutic options available for psoriasis, there remains a significant need for safe and effective therapies,” said April Armstrong, associate dean and professor of dermatology at the University of Southern California.

XTalks
APRIL 17, 2023
These results are very promising for patients with atopic dermatitis,” said Jonathan Silverberg, MD, professor of dermatology at George Washington University School of Medicine & Health Sciences and co-investigator of the studies, in the company’s press release. ADvocate2 showed similar highly statistically significant results.

pharmaphorum
FEBRUARY 28, 2022
Amryt Pharma has been handed a major blow by the FDA, after the US regulator rejected its marketing application for Oleogel-S10, its drug for rare and debilitating skin disease epidermolysis bullosa (EB). The post FDA turns down Amryt’s epidermolysis bullosa drug appeared first on.

pharmaphorum
SEPTEMBER 2, 2022
Spevigo is the first approved therapy for GPP, and the first drug in the IL-36 inhibitor class to make it through development and onto the market. The drug has however just failed a phase 2 test in moderate-to-severe hidradenitis suppurativa, another skin disorder, and has been dropped for that indication.

Pharmaceutical Technology
FEBRUARY 28, 2023
According to GlobalData, Phase I drugs for Malignant Mesothelioma have a 77% phase transition success rate (PTSR) indication benchmark for progressing into Phase II. GlobalData’s report assesses how KFA-115’s drug-specific PTSR and Likelihood of Approval (LoA) scores compare to the indication benchmarks.

pharmaphorum
DECEMBER 15, 2021
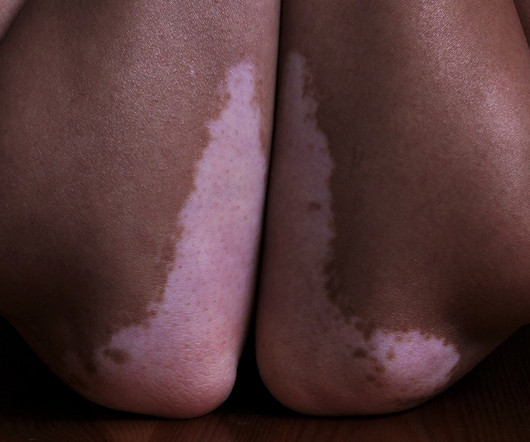
If approved, Opzelura would provide an alternative to steroid drugs currently used to treat vitiligo, a chronic autoimmune disease that causes depigmentation of skin and affects around 1.5 The post FDA sets April date for verdict on Incyte’s vitiligo drug appeared first on. million people in the US.

Drug Channels
JULY 11, 2022
Drug Channels readers will save 10% off the current registration rate when they use code 22DC10 *. Drug Channels readers will save 10% off the current registration rate when they use code 22DC10 *. Drug Channels, or any of its employees. d/b/a Drug Channels Institute. Delivered as a Hybrid Event.

The Pharma Data
OCTOBER 29, 2020
30, 2020 /PRNewswire/ — Eli Lilly and Company (NYSE: LLY) and Incyte (NASDAQ: INCY) announced today new data for baricitinib (marketed as OLUMIANT ® ) will be presented at the annual Fall Clinical Dermatology meeting taking place virtually October 29-November 1, 2020. INDIANAPOLIS , Oct.
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?


Let's personalize your content