Verrica’s Dr. Lawrence Eichenfield Speaks About FDA Approval of New Drug for Common Skin Infection – Xtalks Life Science Podcast Ep. 121
XTalks
AUGUST 2, 2023
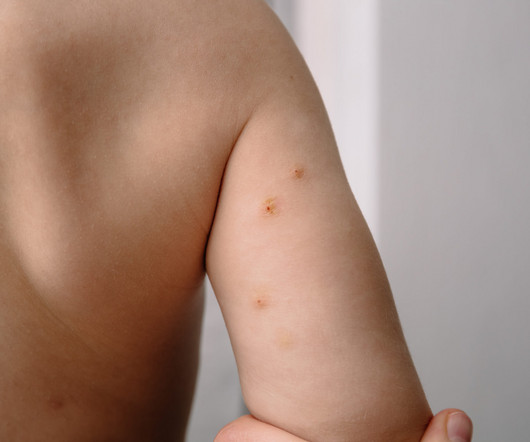
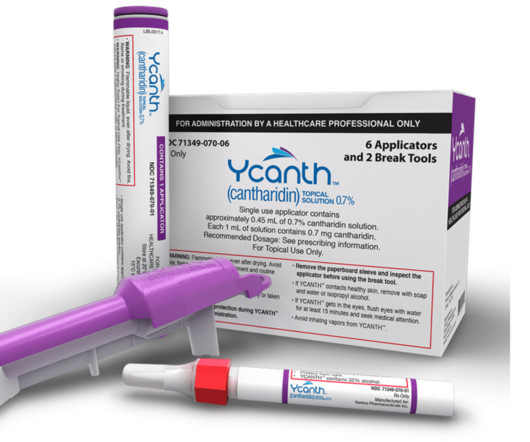
Eichenfield serves on Verrica Pharmaceuticals’ Board of Directors and spoke to Xtalks about the company’s recent approval of YCANTH (cantharidin) topical solution as the first FDA approved treatment for pediatric and adult patients with molluscum contagiosum, a highly contagious viral skin infection that primarily affects children.





















Let's personalize your content