FDA hands Cassiopea its first approval, for acne drug Winlevi
pharmaphorum
AUGUST 28, 2020
Shares in dermatology specialist Cassiopea, which is listed on the SWX exchange in Switzerland, rose almost 17% after news of the FDA approval emerged.

pharmaphorum
AUGUST 28, 2020
Shares in dermatology specialist Cassiopea, which is listed on the SWX exchange in Switzerland, rose almost 17% after news of the FDA approval emerged.

pharmaphorum
DECEMBER 1, 2021
Current treatments are dominated by generic drugs, and include topical antiseptics, retinoids which work by removing dead skin cells from the surface of the skin but can cause pain and inflammation, and topical antibiotics, as well as hormonal therapies including oral androgen inhibitors like spironolactone or cyproterone.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

pharmaphorum
OCTOBER 26, 2022
Food and Drug Administration approved Vtama (tapinarof) cream, an aryl hydrocarbon receptor agonist and the first FDA-approved steroid-free topical medication in its class. Two months later, Arcutis won approval for its Zoryve (roflumilast) cream. When this hormone is suppressed, response to stressors (e.g.
The Pharma Data
AUGUST 17, 2021
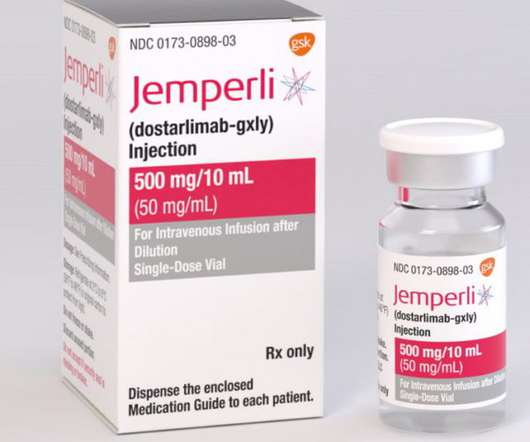
Second FDA approved indication for dostarlimab in 2021 GARNET study demonstrated objective response rate of 41.6% This indication received accelerated approval based on tumour response rate and durability of response. This approval was based on data from cohort A1, which included 71 patients with dMMR endometrial cancer.
The Pharma Data
JULY 23, 2021
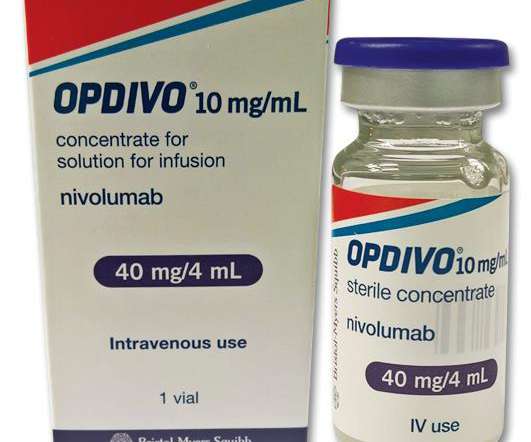
1%) as determined by an FDA-approved test, with no EGFR or ALK genomic tumor aberrations. Patients with EGFR or ALK genomic tumor aberrations should have disease progression on FDA-approved therapy for these aberrations prior to receiving OPDIVO. endocrinopathies and dermatologic reactions) are discussed below.
The Pharma Data
MARCH 11, 2021
1%) as determined by an FDA-approved test, with no EGFR or ALK genomic tumor aberrations. Patients with EGFR or ALK genomic tumor aberrations should have disease progression on FDA-approved therapy for these aberrations prior to receiving OPDIVO. endocrinopathies and dermatologic reactions) are discussed below.

XTalks
AUGUST 2, 2023
Paxlovid was first authorized under the FDA Emergency Use Authorization in December 2021 ; however, it has received FDA approval on May 25, 2023. pneumoniae serotypes) replaced the company’s first pneumococcal conjugate vaccine Prevnar (PCV7, approved by the FDA in February 2000) in a February 2010 FDA approval.
Let's personalize your content