Regenerative medicine: new focus on VEGF target?
Pharmaceutical Technology
MARCH 27, 2023
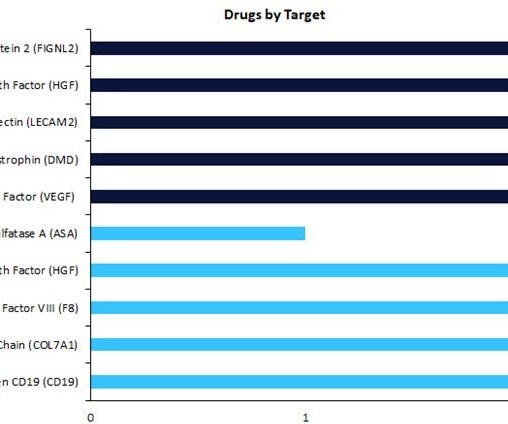
Regenerative medicines in early-stage development (preclinical, discovery, or investigational new drug [IND]/ clinical trial application [CTA] filed status) have seen a change in drug targets compared to therapies in late-stage development (Phase II to pre-registration stage).






































Let's personalize your content