Incyte stock hit 11% despite paediatric dermatology trials hitting target
Pharmaceutical Technology
MARCH 17, 2025
The two simultaneous Incyte trials in HS saw more than 40% of patients on povorcitinib achieving a clinical response.
This site uses cookies to improve your experience. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country, we will assume you are from the United States. Select your Cookie Settings or view our Privacy Policy and Terms of Use.
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.
Used for the proper function of the website
Used for monitoring website traffic and interactions
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.

Pharmaceutical Technology
MARCH 17, 2025
The two simultaneous Incyte trials in HS saw more than 40% of patients on povorcitinib achieving a clinical response.

XTalks
NOVEMBER 7, 2024
Emrosi’s approval was based on data from a pair of Phase III clinical trials for the treatment of rosacea. The trials met all co-primary and secondary endpoints, with participants having successfully completed the 16-week treatment with no significant safety issues.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

XTalks
OCTOBER 24, 2024
In August 2024, Artax Biopharma completed patient recruitment for its Phase IIa trial of AX-158, exploring a new immune-modulating therapy for psoriasis. In this blog, we break down some of the latest innovations in psoriasis treatment, including biosimilars, AI-driven diagnostics and the growing role of family care.

BioPharma Reporter
DECEMBER 14, 2023
UK-based Lindus Health and US-based Thirty Madison today (December 14) announced they have completed the enrolment of a trial to assess their personalized dermatology telemedicine platform, Facet.

Pharmaceutical Technology
OCTOBER 25, 2023
The funds will go towards Phase II trials for the company’s two candidates treating seborrheic keratoses and melasma.

Pharma Times
APRIL 12, 2023
The trial will monitor patients across Europe for 24 months as they are treated with tildrakizumab

Pharma Times
MARCH 10, 2023
Collaboration will concentrate on launching and running studies within medical dermatology

Rethinking Clinical Trials
JUNE 26, 2024
Mirza is chief resident in the Department of Dermatology at the Warren Alpert Medical School of Brown University. The post June 26, 2024: Using ChatGPT to Facilitate Informed Medical Consent, in This Week’s PCT Grand Rounds appeared first on Rethinking Clinical Trials. Join the online meeting.

Pharmaceutical Technology
JULY 12, 2022
A validated target in dermatology, PDE4 is an enzyme that induces overactive immune responses. The NDS submission by Arcutis is based on positive findings from the company’s pivotal Phase III programme and two long-term open-label clinical trials. Nearly one out of four subjects in these two trials were enrolled in sites in Canada.

pharmaphorum
JULY 5, 2022
Trial organisers face intense competition to find and recruit eligible patients for atopic dermatitis studies. With more than 500 dermatology clinical trials currently underway, it is often heard that there is a ‘’competition for participants’’. Patient recruitment for moderate or severe atopic dermatitis.

Pharmaceutical Technology
APRIL 13, 2023
Following the increased use of telemedicine during the Covid-19 pandemic, the potential of digital technologies in communication, data collection, and analysis has become increasingly realised by patients, healthcare systems, and clinical trial sponsors.

WCG Clinical
OCTOBER 9, 2023
The dermatology market is embarking on tremendous growth and sponsors need to be ready. Over the last 12 months, dermatology-focused studies have increased by roughly 20%, with top focus areas including psoriasis, atopic dermatitis, and alopecia. This rapid growth brings new challenges. Efficiency and speed are critical.

FDA Law Blog
NOVEMBER 10, 2024
Each year, FDA selects a limited number of clinical trials to fund to help sponsors pursue development of medical products for rare diseases and advance their field. In October, FDA announced seven new clinical trial grants awarded in fiscal year (FY) 2024 – including one for a Phase 3 trial – totaling $17.2

Pharmaceutical Technology
JANUARY 19, 2023
Concert’s patent portfolio includes oral Janus kinases JAK1/2 inhibitor, deuruxolitinib, to treat Alopecia Areata, an autoimmune dermatological disease. It is currently being evaluated in two open label, long-term extension trials in North America and Europe.

Pharmaceutical Technology
MAY 19, 2023
The FDA nod means Rinvoq is now approved for seven indications in gastroenterology, rheumatology and dermatology. Based on the clinical trial results, treatment with Rinvoq shows both early and long-term symptom relief along with evidence of a visible reduction of damage to the intestinal lining caused by excess inflammation,” said Dr.

BioSpace
APRIL 21, 2021
On the back of the two positive Phase III trials, Ortho said it will submit a new drug application (NDA) to the U.S. Food and Drug Administration (FDA) in the second half of 2022.

Fierce Pharma
OCTOBER 25, 2024
Key drugs from Johnson & Johnson and Eli Lilly stand to bolster their respective cases in competitive dermatology markets with new data readouts.
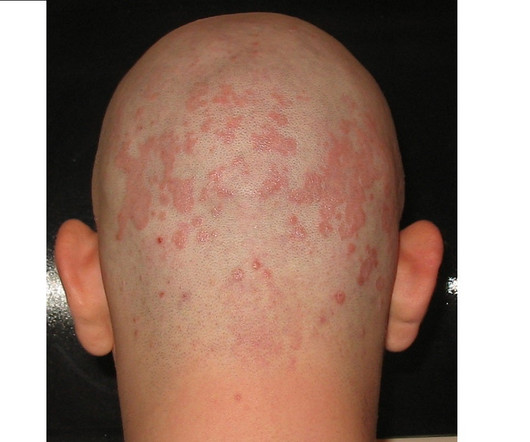
Pharmaceutical Technology
APRIL 19, 2023
“If approved, roflumilast foam would be the first topical drug with a new mechanism of action for this condition in over two decades, highlighting the unique formulation and deep dermatological expertise that Arcutis brings to immuno-dermatology.” The findings showed that the trial met its primary endpoint, with a 79.5%

Velocity Clinical Research
JULY 18, 2023
Born and trained in Egypt, he moved to the UK several years ago after working as a general practitioner there and deciding he loved the country and the clinical trial opportunities it offered. To join a clinical trial at Velocity, visit velocityclinicaltrials.com. By 2018, she was directing seven sites.

XTalks
AUGUST 14, 2024
The approval marks a huge milestone for nemolizumab, which recently demonstrated significant itch-relief in clinical trials last month in adolescent and adult patients with moderate-to-severe atopic dermatitis. A significant percentage of patients also reported reduced sleep disturbances by Week 16 in both clinical trials.
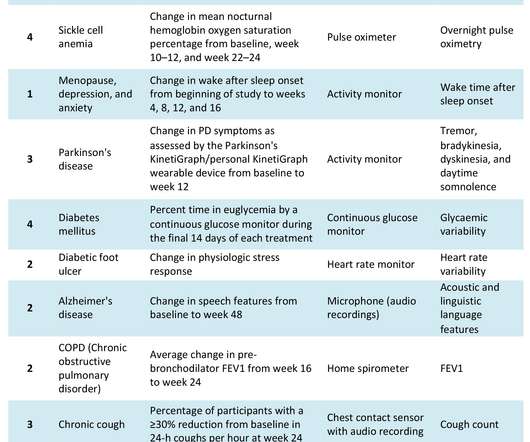
pharmaphorum
JULY 8, 2022
The COVID-19 pandemic has catalysed significant changes in the way pharma develops drugs, particularly in the clinical trial space. Hybrid or decentralised clinical trials (DCTs) have gained traction as technology, infrastructure and knowledge have evolved to support their use. New sensor technology revolutionising data collection.
STAT News
DECEMBER 2, 2022
And a fragmented clinical trials industry has made it a prime target for private equity, which often consolidates markets by merging companies.

WCG Clinical
SEPTEMBER 20, 2023
Welcome to the recorded session of ‘Optimizing Endpoint Assessments for Dermatology Studies.’ In this insightful webinar, we take a deep dive into dermatology trials and discuss how standardizing rater qualification and training can solve common challenges and protect the quality and integrity of endpoint data.

XTalks
MAY 19, 2023
Clinical trials play a vital role in advancing patient treatment and medical care. This article delves into ten trends and statistics in the world of clinical trials for 2023. And don’t forget to explore the upcoming webinars about clinical trials on Xtalks to see what piques your curiosity about the latest clinical trial solutions.

Pharmaceutical Commerce
JANUARY 16, 2024
Zoryve (roflumilast) shows efficacy in seborrheic dermatitis and atopic dermatitis in clinical trials.

VirTrial
JULY 7, 2024
In recent years, there has been significant activity in the AD clinical trial landscape, including the approval of novel systemic therapies. Accurate measurement of AD severity over time is crucial for evaluating treatment efficacy in these trials. In these ways, the two assessments measure BSA quite differently.

Pharmaceutical Technology
FEBRUARY 28, 2023
Attributes of the drug, company and its clinical trials play a fundamental role in drug-specific PTSR and likelihood of approval. GlobalData tracks drug-specific phase transition and likelihood of approval scores, in addition to indication benchmarks based off 18 years of historical drug development data.
WCG Clinical
MARCH 30, 2023
Uniform Live Action Training Using InvestigatorSpace Speeds Up Study Start Times In one case, a global pharmaceutical company used WCG’s training solutions to support two dermatology studies they were running. Study teams engaged with the training content throughout the trial and asked questions through the InvestigatorSpace platform.

Fierce Pharma
OCTOBER 13, 2023
Now, the Danish dermatology specialist is peeling back the layers on those results.

XTalks
MARCH 18, 2025
Incytes recent announcement on the topline results from two Phase III clinical trials of povorcitinib in patients with hidradenitis suppurativa has stirred both hope and caution in the market. These promising figures had set high expectations for subsequent trials. The other Phase III trial, STOP-HS2, showed a 42.3% for placebo.

Outsourcing Pharma
FEBRUARY 2, 2021
While the technology has been found mostly in dermatological applications, its use in studies is expanding, according to Illingworth Research Group.

XTalks
APRIL 17, 2023
In clinical trials, patients experienced significantly clearer skin and less interference with sleep due to itch when taking lebrikizumab compared to placebo. The ADvocate1 and ADvocate2 Phase III studies were published in the New England Journal of Medicine and the British Journal of Dermatology , respectively.

VirTrial
JULY 7, 2024
In recent years, there has been significant activity in the AD clinical trial landscape, including the approval of novel systemic therapies. Accurate measurement of AD severity over time is crucial for evaluating treatment efficacy in these trials. In these ways, the two assessments measure BSA quite differently.

VirTrial
JULY 7, 2024
In recent years, there has been significant activity in the AD clinical trial landscape, including the approval of novel systemic therapies. Accurate measurement of AD severity over time is crucial for evaluating treatment efficacy in these trials. In these ways, the two assessments measure BSA quite differently.
Pharmaceutical Technology
APRIL 17, 2023
The company will provide regulatory advice to Incannex, and manage clinical trials to develop CannQuit and ReneCann products to treat addiction and immune-disordered skin diseases. The company will help in managing the clinical trials of the products once the regulators provide approvals for the proposed research and development programmes.

BioTech 365
JANUARY 15, 2022
Dermavant Presents New Patient Satisfaction Data from PSOARING 3 Long Term Extension Trial of Tapinarof in Adults with Plaque Psoriasis at the 2022 Winter Clinical Dermatology Conference Dermavant Presents New Patient Satisfaction Data from PSOARING 3 Long Term Extension Trial … Continue reading →

Fierce Pharma
MARCH 11, 2024
The disease setting currently has no approved treatment options.

Delveinsight
SEPTEMBER 28, 2021
Innovent, UNION Therapeutics Set to Advance Dermatology Market in China. Innovent Biologics and UNION therapeutics have announced a strategic and licensing collaboration to develop and commercialize Orismilast , a next-generation PDE4 inhibitor for inflammatory dermatology conditions in China.

BioTech 365
SEPTEMBER 23, 2020
23, 2020 (GLOBE NEWSWIRE) — Verrica Pharmaceuticals Inc. (“Verrica”) (Nasdaq: VRCA), a dermatology therapeutics company developing medications for skin diseases requiring medical interventions, today announced that positive results from the two pivotal Phase III CAMP (Cantharidin … Continue reading →

XTalks
SEPTEMBER 30, 2024
Presented at the European Academy of Dermatology and Venereology (EADV) Congress 2024, the study highlighted significant improvements in key disease markers, positioning orismilast as a potential breakthrough in atopic dermatitis management.
pharmaphorum
SEPTEMBER 16, 2022
Atopic dermatitis (AD) is a highly prevalent chronic dermatological disease, with symptoms which include itching, dry skin, severe pain, and inflammation – all of which create considerable stress in the daily lives of patients and often negatively affect their day-to-day and long-term well-being. However, side effects do happen.

Rethinking Clinical Trials
SEPTEMBER 24, 2024
Mirza, MD, MPH Chief Resident Department of Dermatology Warren Alpert Medical School of Brown University Slides Keywords Artificial Intelligence; ChatGPT; Informed Consent Key Points Artificial Intelligence (AI), when implemented thoughtfully in clinical settings, can lead to meaningful results for patients.

VirTrial
JULY 7, 2024
In recent years, there has been significant activity in the AD clinical trial landscape, including the approval of novel systemic therapies. Accurate measurement of AD severity over time is crucial for evaluating treatment efficacy in these trials. In these ways, the two assessments measure BSA quite differently.
XTalks
AUGUST 2, 2023
Eichenfield was also the principal investigator on clinical trials evaluating YCANTH. Verrica Pharmaceuticals is a dermatology therapeutics company developing medications for skin diseases that require medical interventions.
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?


Let's personalize your content