Scribe and Sanofi expand genetic therapy development deal
Pharmaceutical Technology
JULY 18, 2023
Scribe Therapeutics and Sanofi have expanded partnership to progress the development of in vivo genetic therapies to treat genomic diseases.
This site uses cookies to improve your experience. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country, we will assume you are from the United States. Select your Cookie Settings or view our Privacy Policy and Terms of Use.
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.
Used for the proper function of the website
Used for monitoring website traffic and interactions
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.

Pharmaceutical Technology
JULY 18, 2023
Scribe Therapeutics and Sanofi have expanded partnership to progress the development of in vivo genetic therapies to treat genomic diseases.

Camargo
NOVEMBER 11, 2020
Each month, Camargo’s “In the News” series highlights important changes and advancements in the regulatory and development space and explores how those changes could impact your program. Unpacking the (Black) Box: Antares Licenses Urology Product with Boxed Warning. of hyponatremia, or low blood sodium levels. In October, the U.S.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
Pharmaceutical Technology
SEPTEMBER 29, 2022
Scribe Therapeutics and Sanofi have signed a strategic partnership to expedite the development of breakthrough clustered regularly interspaced short palindromic repeats (CRISPR)-based cell therapies for cancer. Scribe is also entitled to receive potential payments worth over $1bn on meeting development and commercial milestones.
Pharmaceutical Technology
FEBRUARY 15, 2023
Innovation S-curve for the pharmaceutical industry CRISPR nuclease is a key innovation area in pharmaceutical development CRISPR, which refers to clustered regularly interspaced short palindromic repeats, are bacteriophage-derived DNA sequences that had previously infected the prokaryote and are found in the genomes of bacteria and archaea.

Pharmaceutical Technology
FEBRUARY 23, 2023
Moderna has entered a strategic research and development partnership with ElevateBio’s Life Edit Therapeutics to discover and develop new in-vivo mRNA gene editing therapies. Moderna will also assume further development, manufacturing, and commercialisation responsibilities on exercising a target option.

pharmaphorum
JANUARY 29, 2021
Genome editing is an exciting but still nascent field, and companies in the area face as many obstacles as they do opportunities. They wanted someone who had lots of experience in drug development, was a molecular biologist, and was stubborn enough to take on CRISPR!” Zinc fingers. billion in funding.

Pharmaceutical Technology
MAY 17, 2023
Scribe Therapeutics has entered a strategic collaboration with Eli Lilly and Company subsidiary Prevail Therapeutics for accelerating in vivo CRISPR-based therapies to target the causes of serious neurological and neuromuscular diseases. The company is also eligible for development and commercial milestone payments exceeding $1.5bn.

pharmaphorum
JUNE 23, 2022
Novartis has shouldered its way into the in vivo gene editing category via a deal with US biotech Precision BioSciences, focused on a therapy for sickle cell disease (SCD). billion in potential milestones if the project advances through development and onto the market. billion agreement that started in 2020.

XTalks
OCTOBER 20, 2023
Clinical-stage genome editing company Intellia Therapeutics has received clearance from the US Food and Drug Administration (FDA) for its Investigational New Drug (IND) application to start a pivotal phase III trial of NTLA-2001 for the treatment of transthyretin (ATTR) amyloidosis with cardiomyopathy.

Pharmaceutical Technology
FEBRUARY 23, 2023
DNA binding site prediction, peptide structure optimisation, and AI-assisted genome analysis are some of the accelerating innovation areas, where adoption has been steadily increasing. However, not all innovations are equal nor do they follow a constant upward trend.

XTalks
AUGUST 5, 2020
Single cell RNA sequencing technology has been extensively applied to understand heterogeneity among tumor cells, within the tumor microenvironment and during cell development. Single cell sequencing has revolutionized the study of biological tissues and systems at the cellular and molecular level. The Power of Single Cell Technology.

Pharmaceutical Technology
FEBRUARY 15, 2023
They are engineered to cut specific genomic targets in order to modify the expression of single genes and proteins. According to GlobalData, there are 190+ companies, spanning technology vendors, established pharmaceutical companies, and up-and-coming start-ups engaged in the development and application of gene-splicing using nucleases.
Scienmag
NOVEMBER 16, 2020
Wnt-regulated lncRNA discovery enhanced by in vivo identification and CRISPRi functional validation. Genome Med 12, 89 (2020). Singapore scientists uncover potential role of long non-coding RNAs in pancreatic cancer Credit: From Figure 4 in Liu, S., Harmston, N., Glaser, T.L.

Roots Analysis
FEBRUARY 27, 2024
The global genome editing market is anticipated to grow at a CAGR of 12.6% The global genome editing market is anticipated to grow at a CAGR of 12.6% The biopharmaceutical pipeline includes close to 500 gene therapies , several of which are being developed based on CRISPR technology. during the forecast period 2023-2035.
Pharmaceutical Technology
FEBRUARY 14, 2023
These systems include human and mouse cell lines, and even in vivo in live animals. These systems include human and mouse cell lines, and even in vivo in live animals. However, not all innovations are equal and nor do they follow a constant upward trend.

XTalks
DECEMBER 20, 2023
As we step into 2024, the life sciences continue to evolve at an unprecedented pace, driven by technological innovation, a deeper understanding of human biology and the application of new technologies in areas like drug development and health wearables. Regulatory bodies are also taking note of the applications of AI in drug development.

pharmaphorum
JULY 13, 2022
Verve Therapeutics has started dosing patients in a phase 1b trial of its in vivo gene-editing drug for high cholesterol, designed to permanently switch off the PCSK9 gene with a one-shot treatment. dosing of *first patient* with VERVE-101, an in vivo CRISPR base editing medicine. Today: we are announcing.

Pharmaceutical Technology
MARCH 9, 2023
Innovation S-curve for the pharmaceutical industry Transgenic murine models is a key innovation area in pharmaceutical s Transgenic murine models refer to mice that have been genetically altered for the purposes of understanding the in vivo functions of genes.

The Pharma Data
DECEMBER 13, 2020
Nasdaq: DTIL) a clinical stage biotechnology company dedicated to improving life with its novel and proprietary ARCUS® genome editing platform, today announced that Abid Ansari, Chief Financial Officer, notified the Company that he will be leaving the organization after nearly five years to pursue a new career opportunity. “On DURHAM, N.C.,

The Pharma Data
JANUARY 12, 2021
Working alongside KSQ will facilitate smart drug discovery and development of what we hope will be transformative new therapies for patients with intractable forms of cancer.”. In addition, KSQ will be eligible for additional option payments along with development and commercialization milestone payments. sales for that product.

The Pharma Data
DECEMBER 13, 2020
which develops genome editing technologies to accelerate drug discovery and develop novel therapeutics for a broad range of diseases, today announced the appointment of Bo Zhang, Ph.D., as Head of Business Development. .–( BUSINESS WIRE )– EdiGene, Inc. as Head of the US Subsidiary, and Kehua Fan, M.D.,

The Pharma Data
DECEMBER 2, 2020
” VANCOUVER, BC, December 02, 2020 /24-7PressRelease/ — Eyam Vaccines and Immunotherapeutics (EYAM) today announced that former President and CEO of Genome Prairie is joining EYAM. Pontarollo’s primary areas of research and technical expertise include Genomics, Molecular Biology, Vaccine development and Immunology.

pharmaphorum
OCTOBER 6, 2022
A complex range of factors are thought to contribute to the development of the illness. After developing a conventional disease pathway model for GWI, researchers then used Elsevier’s data and technology to create a model to act as a basis for in silico modelling of the disease. Disease modelling Gulf War Illness.

XTalks
MAY 22, 2024
In this episode, Ayesha spoke with John Finn, PhD, Chief Scientific Officer at Tome Biosciences , a company developing programmable gene insertion (PGI) technology. PGI is a revolutionary approach for the development of potentially curative cell and integrative gene therapies.

The Pharma Data
JANUARY 10, 2021
Nasdaq: EDIT), a leading genome editing company, today announced the U.S. The Company is required to develop and submit to the FDA an improved potency assay prior to enrolling the efficacy phase of the RUBY trial. EDIT-301 is an experimental, ex vivo gene editing cell medicine in development for the treatment of sickle cell disease.

pharmaphorum
OCTOBER 8, 2020
Scribe – which counts new Nobel Prize for chemistry winner Jennifer Doudna among its founders – is getting $15 million upfront from Biogen for an alliance focusing on the development of a CRISPR-based therapy for neurodegenerative disease including amyotrophic lateral sclerosis (ALS). Benjamin Oakes.
The Pharma Data
NOVEMBER 22, 2020
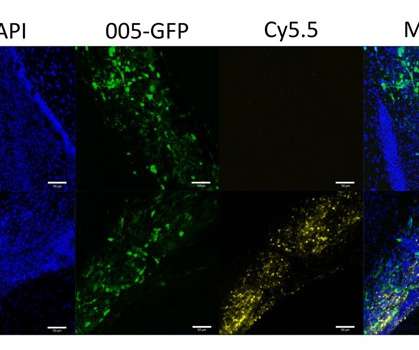
Peer stated that his team plans to develop a treatment for all cancers and that their technique could be ready for humans within the next two years. CTX001 is being developed under a co-development and co-commercial agreement between the two organizations. Photo courtesy of Science Advances.

The Pharma Data
JUNE 7, 2023
a biotechnology company specializing in the development of lipid nanoparticle (LNP) delivery systems for molecular therapeutics. Acuitas’ LNP technology will support Bayer’s in vivo gene editing and protein replacement programs by specifically delivering RNA payloads to the desired target organ, the liver.

Delveinsight
FEBRUARY 25, 2021
Instead of snipping the genome, base editing lets for edits of individual letters in a genetic sequence. Electroporation is used for ex vivo delivery of therapies to blood and immune cells. GuideTx will aid Beam in developing LNP-based medicines. Beam employs three approaches to deliver its genetic medicines to cells.

XTalks
APRIL 24, 2023
Rare diseases can often be progressive, chronic and fatal. Approximately 72 percent of rare diseases are genetic, and around 70 percent of rare genetic diseases emerge in childhood. Sadly, one-third of children with rare diseases die before their first birthday.

The Pharma Data
DECEMBER 1, 2020
NASDAQ:NTLA), a leading genome editing company focused on developing curative therapeutics using CRISPR/Cas9 technology both in vivo and ex vivo , announced today the pricing of an underwritten public offering of 4,794,521 shares of its common stock at a public offering price of $36.50 CAMBRIDGE, Mass.,

Pharma Marketing Network
DECEMBER 21, 2020
Almost two decades after the human genome was sequenced, a trickle of new genetic medicines (i.e., those that modify the expression of an individual’s genes or repair abnormal genes) has entered clinical practice, including 11 RNA therapeutics, 2 in vivo gene therapies, and 2 gene-modified cell therapies.

XTalks
NOVEMBER 3, 2023
CRISPR works as genetic scissors to edit parts of the genome. The scientists on the advisory committee were particularly focused on the CRISPR gene editing technology itself and how companies like Vertex that are developing CRISPR-based therapies are ensuring their treatments are not making off-target gene edits.

The Pharma Data
OCTOBER 14, 2020
Priothera will use the funds to progress the clinical development of mocravimod, a modulator of sphingosine 1 phosphate (S1P) receptors, to enhance the curative potential of allogeneic hematopoietic stem cell transplantation (HSCT) for treating AML. All jobs have been maintained and the workforce has grown from about 220 to 350.

Pharma Marketing Network
JANUARY 20, 2021
In other areas of the industry (such as cell and genetic medicine development) progress continued at an impressively brisk pace despite the pandemic. Regarding OWS’s support for vaccine development, Dr Slaoui noted that 5 of the 6 vaccines selected (from a total of 94 programs) are currently in phase 3 development or approved.
The Pharma Data
DECEMBER 10, 2020
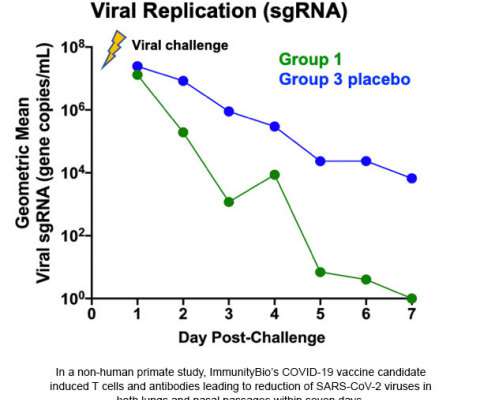
Many people who have been exposed to the “common cold” potentially develop adenovirus immunity: the immune system often attacks and disables these first-generation vaccines before they can activate the immune response to attack the SARS-CoV-2 virus. This blocking of viral replication was observed in both the lung and nasal passages.

The Pharma Data
JANUARY 4, 2021
Kiromic chPD1 has shown in preclinical data to show a cytotoxic response in 9 different in vivo models with 100% long-term PFS with the induction of host memory responses. PD-1 has always been a challenge for CAR-T development. “We believe Prof. About PD-1 Check-point inhibition. Dr. Barber is a tumor immunologist.

XTalks
AUGUST 12, 2020
The identification of T cells that essentially ‘hide’ the virus has significant implications for developing therapeutic strategies to improve the treatment and long-term management of HIV. This may help explain why HIV is adequately controlled, but not eradicated, with current treatments. The research study was published in Cell Reports.

FDA Law Blog
DECEMBER 6, 2024
Valentine On November 19, 2024, FDA released a draft guidance titled Frequently Asked Questions Developing Potential Cellular and Gene Therapy Products. academic to industry) involved in developing CGTs, the guidance starts by summarizing some of the fundamentals of opening an IND.

Roots Analysis
JANUARY 27, 2025
Most of the companies offer protein expression for research applications, due to their extensive use in early-stage discovery, lead candidate screening and in vivo studies. Considering their vast potential, close to 50% of the top selling drugs in 2023 were protein therapeutics.
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?


Let's personalize your content