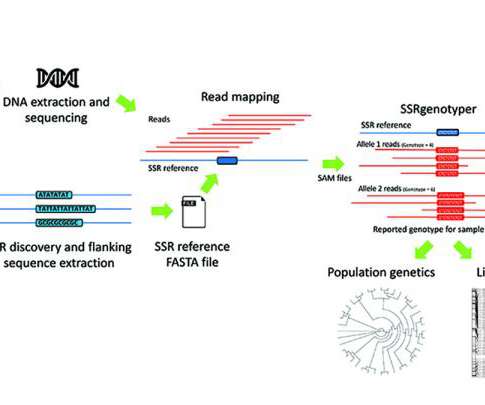
SSRgenotyper: A new tool to digitally genotype simple sequence repeats
Scienmag
FEBRUARY 4, 2021
SSRgenotyper: A simple sequence repeat genotyping application for whole-genome resequencing and reduced representational sequencing projects.
This site uses cookies to improve your experience. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country, we will assume you are from the United States. Select your Cookie Settings or view our Privacy Policy and Terms of Use.
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.
Used for the proper function of the website
Used for monitoring website traffic and interactions
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.
Scienmag
FEBRUARY 4, 2021
SSRgenotyper: A simple sequence repeat genotyping application for whole-genome resequencing and reduced representational sequencing projects.

Medical Xpress
APRIL 3, 2023
Work by an international research team led by Yu Xu and Michael Inouye at the Department of Public Health and Primary Care, University of Cambridge, has resulted in a unique resource for predicting multi-omics data directly from genotypes. A Research Briefing on the study is published in the same journal issue.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
Pharmaceutical Technology
JULY 4, 2022
Brii Biosciences (Brii Bio) has exercised an option for the acquisition of exclusive development and marketing rights for Vir Biotechnology’s investigational antibody, VIR-3434, for Hepatitis B in Greater China, under a partnership agreement. The mAb is presently in the Phase II development stage.
Pharmaceutical Technology
AUGUST 18, 2022
0 genotypes. After more than a decade of research and clinical development, and through the perseverance of clinicians, patients and their families, the approval of Zynteglo marks a watershed moment for the field of gene therapy.”. Findings showed that 89% of evaluable subjects attained transfusion independence (TI).

Medical Xpress
FEBRUARY 28, 2023
Using artificial intelligence (AI) methods, researchers led by Professor Dr. Alexander Schönhuth from Bielefeld University's Faculty of Technology have succeeded in recording and deciphering the genotype profiles of 3,000 ALS patients and thus learning more about the development of the disease.

Pharmaceutical Technology
SEPTEMBER 5, 2022
The treatment is indicated for CF patients who are homozygous for the F508del mutation (F/F genotype) in the cystic fibrosis transmembrane conductance regulator (CFTR) gene. It enrolled 46 children aged one to under two years with the F/F genotype. According to the findings, Orkambi was found to be well tolerated.

BioTech 365
OCTOBER 5, 2021
SpeeDx Build Further Coverage in SARS-CoV-2 Genotyping Reagent Portfolio SpeeDx Build Further Coverage in SARS-CoV-2 Genotyping Reagent Portfolio Rapid development of key mutation detection reagents continues to facilitate critical investigation and monitoring activities SYDNEY–(BUSINESS WIRE)–#COVID19–SpeeDx Pty.

BioTech 365
FEBRUARY 5, 2021
Biotechnology, Pharma and Biopharma News – Research – Science – Lifescience ://Biotech-Biopharma-Pharma: SSRgenotyper: A new tool to digitally genotype simple sequence repeats.SSRgenotyper is a newly developed, free bioinformatic tool that allows researchers to digitally genotype sequenced populations using simple … Continue (..)

Worldwide Clinical Trials
AUGUST 14, 2024
Hematological cancer cells can also develop resistance to therapies over time, reducing treatment effectiveness. These developments, combined with targeted therapies, have dramatically improved survival status and quality of life for those diagnosed with hematological cancers.

Worldwide Clinical Trials
FEBRUARY 6, 2023
The best course of action will depend on many variables, such as the disease or population being studied and the genotype/phenotype relationship, risk, and impact on care. Obtaining appropriate assistance to facilitate planning up front for the complexities involved will save sponsors time and effort in the long run.

Scienmag
AUGUST 25, 2020
Credit: Iliana Baums, Penn State Coral conservation efforts could get a boost from a newly developed genotyping “chip”–the first of its kind for corals.
XTalks
JANUARY 21, 2025
TriClip G4 System Manufacturer/developer : Abbott Medical Date of FDA approval : April 1, 2024 Approved for : Tricuspid regurgitation (TR). Abbott Spinal Cord Stimulation (SCS) Systems Manufacturer/developer : Abbott Medical Date of FDA approval : May 30, 2024 Approved for : Chronic, hard-to-manage pain in the torso, arms and legs.

Scienmag
JUNE 23, 2022
Orofacial clefts (OFCs) are congenital birth defects where the independently derived facial primordia that form the orofacial complex fail to fuse properly during embryonic development. 170 GWAS derived SNPs were genotyped on 3000 replication samples from participants in Ethiopia, Ghana, and […].

Scienmag
JANUARY 13, 2021
To discover these associations, researchers need to compare the genomes of many individuals at millions of genetic locations or markers, and therefore require cost-effective genotyping technologies. A new statistical method, developed […].

Pharmaceutical Technology
DECEMBER 15, 2022
Since HFC replacement therapy is the mainstay of treatment for CFD, increased development of more innovative fibrinogen-based therapies will benefit both the CFD population and patients experiencing AFD. CFD is an extremely rare disease in which many patients are often asymptomatic and do not require treatment.

pharmaphorum
JANUARY 21, 2022
The findings have been submitted to the Genotype-to-Phenotype National Virology Consortium (G2P-UK), the New and Emerging Respiratory Virus Threats Advisory Group (NERVTAG) and the Joint Committee on Vaccination and Immunisation (JCVI).

Pharmaceutical Technology
JUNE 6, 2023
The rNPV model is a more conservative valuation measure that accounts for the risk of a drug in clinical development failing to progress. Pharma Mar Overview Pharma Mar discovers, develops and markets marine-derived drugs to treat cancer. Its development pipeline includes PM14 for solid tumors, among others.

BioTech 365
AUGUST 17, 2020
ETD002 is a novel TMEM16A chloride channel potentiator Therapy applicable to all cystic fibrosis patients, independent of genotype BRIGHTON, England–(BUSINESS WIRE)–Enterprise Therapeutics Ltd (Enterprise), a biopharmaceutical company dedicated to the discovery and development of novel therapies to improve the lives … Continue reading (..)

XTalks
MARCH 29, 2021
Grantz, of the Division of Intramural Population Health Research at NIH’s Eunice Kennedy Shriver National Institute of Child Health and Human Development. The results did not differ based on fast or slow caffeine metabolism genotype. Related: Maternal Antibodies: How Allergies Can be Passed from Mothers to Children.

pharmaphorum
JANUARY 24, 2023
Drug development has long been an issue for the pharma industry, due to the expense and the high failure rate of potential treatments. Ben Hargreaves finds that the vast amount of genetic data that exists today could help provide a faster, more targeted way of developing new drug candidates.
Bioengineer
JANUARY 16, 2024
Often, the loss of T790M is associated with the development of alternative competitive resistance mechanisms. Importantly, a triple mutant scenario with EGFR L858R/T790M/C797S or EGFRexon19del/T790M/C797S genotype may occur in patients with T790-positive NSCLC receiving osimertinib in second or later lines.
Worldwide Clinical Trials
DECEMBER 12, 2022
Genetic testing provides patients with a diagnosis for their illness, helps patients and family members to understand risks of developing new diseases, and can be used to support clinical trial advancement. It is important to consider the type of testing performed (e.g., single gene testing vs comprehensive panel), the reportable range (e.g.,
XTalks
APRIL 29, 2021
While ARV-based treatments work well to keep the virus suppressed and patients healthy — some for many years — resistance to one or more drugs in a treatment regimen can develop in some patients. HIV drug resistance occurs when virions develop mutations that make them less susceptible to given drugs. Proviral DNA Genotyping.

pharmaphorum
JANUARY 29, 2019
How the shift towards cancer as a phenotype/genotype is being applied in clinical trials and how they are run •What are co-clinical trials and why could they hold the key to answering unmet need in cancer treatment and beyond into broader drug development? What are the implications from these changes for clinical trials?
XTalks
SEPTEMBER 28, 2020
The test was developed by Resolution Bioscience and the company will run test analyses in its labs. The Resolution ctDx Lung assay was developed to offer patients and clinicians with pertinent information that can inform treatment decisions. Related: Liquid Biopsies Help Match Cancer Patients to Phase I Trials.

The Pharma Data
NOVEMBER 18, 2020
18, 2020 — Genetic variants in some patients with inflammatory bowel disease (IBD) are associated with a significantly increased risk for developing thromboembolic disease (TED), according to a study published online Oct. WEDNESDAY, Nov. 21 in Gastroenterology. Takeo Naito, Ph.D.,
XTalks
FEBRUARY 4, 2021
Data generated through high-throughput omics approaches paired with clinical patient records can give rise to remarkably robust datasets to inform and guide disease insights and development of targeted therapeutics. Genomic biomarker discovery helps drive the development of innovative new personalized therapies. Biobanking Models.

pharmaphorum
AUGUST 11, 2022
First, members who opt-in to the program receive a saliva collection kit, which is analysed using newly developed pharmacogenomics technologies. With this promise beginning to be realised, it’s on drug makers to use genomic testing to steer their development process.
XTalks
SEPTEMBER 15, 2020
A vaccine developed by Swedish company Diamyd Medical has demonstrated significant treatment efficacy in a predefined genetic subgroup of individuals with type 1 diabetes in a Phase IIb clinical trial. Diamyd Medical is a clinical-stage biopharmaceutical company that develops therapies for type 1 diabetes.

pharmaphorum
OCTOBER 9, 2020
Most experience some form of cognitive difficulty, and some develop psychosocial problems related to short stature and feelings of social isolation. The team hopes that this study’s scope will also shed light on the gene’s impact on the women who carry it in addition to an elaborative genotype-phenotype correlation.

The Pharma Data
SEPTEMBER 8, 2021
TIB Molbiol excels in ultra-rapid assay development for emerging infectious disease, strongly demonstrated during the COVID-19 pandemic. For example, in 2001 with anthrax and 2003 with SARS-CoV1, TIB Molbiol demonstrated their ability to develop PCR assays for the detection of new pathogens within days.

Roots Analysis
APRIL 28, 2024
Factors Contributing to Kidney Diseases There are several factors which contribute to an increased risk of developing kidney diseases, including genetic causes and underlying metabolic causes ( diabetes, high blood pressure, aging and obesity ), which when combined with other factors increase the risk of developing kidney diseases.

XTalks
NOVEMBER 10, 2020
” A rare disease diagnosis can also influence family planning options, as well as lead to better development tools, support for patients, and improved patient outcomes. We work with our users and collaborators internationally to develop these systems. ” or “Why is my child different?”

Roots Analysis
AUGUST 12, 2024
Such test can be used for gene expression profiling, genotyping and detecting chromosomal abnormalities. These tests can identify biomarkers, genetic markers, and other molecular indicators that are associated with the development and progression of neurological diseases.
Advarra
FEBRUARY 28, 2023
IRBs may be asked to assist the institution in developing general guidelines for DMS plans, or to participate in pre-review of proposed DMS plans prior to NIH funding application. For example, the Genomic Data Sharing Policy applies to NIH-funded research generating large-scale human or non-human genomic data.

Roots Analysis
SEPTEMBER 20, 2023
Cannabis testing is also done to identify the plant genotype used to develop the product, providing transparency to the consumers. In addition, analyzing the moisture content of the product allows the developers to estimate the product shelf life.

XTalks
DECEMBER 12, 2023
This is crucial not only for understanding these conditions but also for developing targeted therapeutic strategies. To accurately identify these genetic factors, genotyping techniques such as Sanger sequencing, next-generation sequencing (NGS) and copy number variant analysis are used.

Roots Analysis
AUGUST 31, 2023
Recent advances in DNA sequencing technologies have led to significant developments in healthcare-focused research on precision medicine and diagnostics. Given the recent shift from the one-size-fits-all model to a more personalized mode of treatment, there has been a growing interest for identification and adoption of novel techniques.

XTalks
APRIL 15, 2021
Experts from Servier and Genuity Science recently spoke on a webinar about using genomics data to drive drug development in heart failure and identify new targets for novel therapeutics. The reverse could also be true whereby the development of coronary artery disease may impact heart rate. HFrEF vs. HFpEF.

Delveinsight
DECEMBER 13, 2020
Due to hepatitis C, most people develop cirrhosis, or scarring of the liver, before liver cancer; about 5–25% of patients with chronic hepatitis C develop cirrhosis over 10–20 years. Research and development in Hepatitis. 2b) was developed to maintain a steady level of an active drug and reduce the frequency of administration.
pharmaphorum
JANUARY 18, 2023
Pharmaceutical companies and biotechs are also adapting their approaches, launching patient finding and engagement programmes that can start years before clinical trials begin and allow them to run ‘recontact by genotype’ studies that the Resilience Project would have liked to do. Giving participants something in return. About the author.
XTalks
JULY 10, 2024
” The Xpert HCV test’s development was validated through the Independent Test Assessment Program (ITAP), part of the National Institutes of Health’s (NIH) Rapid Acceleration of Diagnostics (RADx) Tech program. Without treatment, over half become chronically infected, with 10 to 20 percent developing cirrhosis over 20 to 30 years.

pharmaphorum
NOVEMBER 10, 2021
This database should support automatic importation from hospitals, laboratories and surveillance networks and link to genotypic data when available.”. New agents: Development and accessibility. This information would support the design and implementation of stewardship programmes.”.

XTalks
APRIL 24, 2023
To achieve this, Medpace works in multi-disciplinary teams of experts to jointly develop and execute advanced therapy protocols. For rare disease studies where there is little or no clinical trial experience, outcome assessments would have to be validated within a clinical development program.
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?


Let's personalize your content