News from AACR 2024: Sunday’s highlights
Drug Discovery World
APRIL 8, 2024
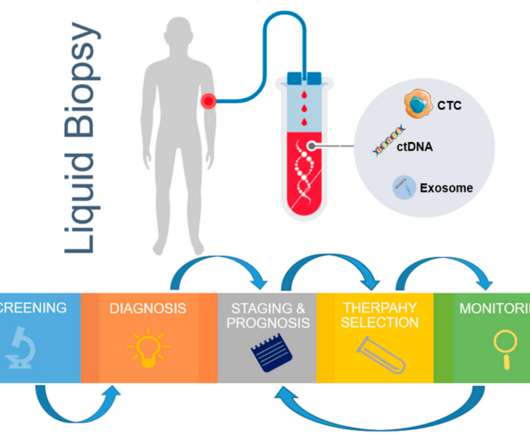
The American Association of Cancer Research (AACR) Annual Meeting launched on Sunday 7 April in San Diego with an Opening Plenary Session showcasing cutting-edge technological advances opening new frontiers in cancer science.
















Let's personalize your content