Astellas teams up with startup Kelonia to make ‘in vivo’ cell therapies
Bio Pharma Dive
FEBRUARY 16, 2024
Kelonia will receive $40 million upfront through a partnership to develop “off-the-shelf” cell therapies for cancer.
This site uses cookies to improve your experience. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country, we will assume you are from the United States. Select your Cookie Settings or view our Privacy Policy and Terms of Use.
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.
Used for the proper function of the website
Used for monitoring website traffic and interactions
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.

Bio Pharma Dive
FEBRUARY 16, 2024
Kelonia will receive $40 million upfront through a partnership to develop “off-the-shelf” cell therapies for cancer.

Pharmaceutical Technology
JULY 18, 2023
Scribe Therapeutics and Sanofi have expanded partnership to progress the development of in vivo genetic therapies to treat genomic diseases.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Bio Pharma Dive
OCTOBER 22, 2024
The gene editing company will focus on “in vivo” medicines, while seeking to license out or find a development partner for its clinical-stage treatment reni-cel.
Pharmaceutical Technology
NOVEMBER 22, 2022
Umoja Biopharma has signed a research agreement with IASO Biotherapeutics (IASO Bio) to develop off-the-shelf therapies for haematological malignancies. The post Umoja and IASO partner to develop therapies for haematological malignancies appeared first on Pharmaceutical Technology.

Bio Pharma Dive
FEBRUARY 22, 2023
The messenger RNA specialist said Wednesday it is teaming up with Life Edit Therapeutics to develop therapies that can modify genes “in vivo.”

Pharmaceutical Technology
OCTOBER 25, 2022
BioMed X has entered a research partnership with Sanofi to leverage artificial intelligence (AI) for drug development. The partnership intends to address a 90% failure rate of new therapy candidates in clinical development, which is a key bottleneck of the pharmaceutical industry.

Bio Pharma Dive
JULY 17, 2023
Scribe will receive $40 million upfront from Sanofi in a collaboration initially focused on developing an in vivo gene editing treatment for sickle cell disease.

Pharmaceutical Technology
APRIL 26, 2024
Regeneron Pharmaceuticals has collaborated with Mammoth Biosciences to develop in vivo clustered regularly interspaced short palindromic repeats (CRISPR)-based gene editing therapies.

Camargo
NOVEMBER 11, 2020
Each month, Camargo’s “In the News” series highlights important changes and advancements in the regulatory and development space and explores how those changes could impact your program. Unpacking the (Black) Box: Antares Licenses Urology Product with Boxed Warning. of hyponatremia, or low blood sodium levels. In October, the U.S.

Pharmaceutical Technology
FEBRUARY 23, 2023
Quotient Sciences helps biotech and pharma customers in the development and optimization of drug products. Our chemists and formulation scientists review the properties of new drug candidates and “work their magic” to develop formulations that improve the exposure profile of the compound.
Pharmaceutical Technology
MAY 19, 2023
Myeloid Therapeutics has raised $73m to support the continued clinical development of its lead cell therapy programme, MT-101, in Phase I/II trials for T cell lymphoma. MT-302 is a TROP2-targeting in vivo chimeric antigen receptor (CAR) that has been designed to express in the myeloid compartment.
Pharmaceutical Technology
APRIL 14, 2023
The company and the researchers, with Professor Krishanu Saha as principal investigator (PI) and Dr Christian Capitini as co-PI, will develop a GD2 CAR T-cell therapeutic candidate to treat neuroblastoma, a cancer type that generally affects young children. Topic sponsors are not involved in the creation of editorial content.

Drug Patent Watch
JANUARY 14, 2025
But how do companies develop these cost-effective alternatives? Generic drug manufacturers are taking a similar approach by developing multiple drugs simultaneously[1]. ”[2] This study not only addressed the ATA’s concerns but also highlighted the power of real-world evidence in generic drug development and regulation. .”[2]

Pharmaceutical Technology
MAY 5, 2023
TFF Pharmaceuticals and the National Institute of Environmental Health Sciences (NIEHS) have signed an agreement for the development of dry powder formulations of high molecular weight hyaluronan (HMW-HA) for respiratory diseases.

Pharmaceutical Technology
MARCH 16, 2023
The partnership will use nanotechnology, which was proven in vivo, for providing antibodies inside tumour cells and provides a potential solution to address the medical challenge. Under the collaboration, Libera Bio and Talem Therapeutics will together develop new antibodies for use with MPN delivery.
Pharmaceutical Technology
JULY 20, 2022
Avista Therapeutics, a University of Pittsburgh Medical Center (UPMC) spinout, has entered a collaboration with Roche for developing new AAV gene therapy vectors for eyes. Avista's computationally steered, in vivo scAAVengr platform uses a high-throughput method with the integrated quantitative validation of new cell-specific AAVs.
Pharmaceutical Technology
NOVEMBER 9, 2022
ProPhase Labs’ wholly owned subsidiary ProPhase BioPharma has entered a two-year collaboration agreement with Dana-Farber Cancer Institute for further research and development of Linebacker-1 (LB-1). LB-1 will be given along with chemotherapy agents in-vitro, in various cell lines, and in-vivo subcutaneous mouse tumour models in the project.

Pharmaceutical Technology
MARCH 24, 2023
Moderna has entered a strategic partnership with Generation Bio for the development of non-viral genetic medicines. The collaboration aims to expand each company’s platform application through the development of new nucleic acid therapeutics, and to expedite their respective non-viral genetic medicines pipelines.
Pharmaceutical Technology
SEPTEMBER 29, 2022
Scribe Therapeutics and Sanofi have signed a strategic partnership to expedite the development of breakthrough clustered regularly interspaced short palindromic repeats (CRISPR)-based cell therapies for cancer. Scribe is also entitled to receive potential payments worth over $1bn on meeting development and commercial milestones.

Pharmaceutical Technology
APRIL 13, 2023
The company is aiming to refocus its efforts on its core therapeutic areas and current late-stage programmes such as the oral TYK2 inhibitor TAK-279, which is under development for several autoimmune disorders.
Pharmaceutical Technology
JANUARY 4, 2023
A synergistic activity was also observed between the anti-Gremlin1 antibody and enzalutamide against castration-resistant prostate cancer models derived from patients in vitro and in vivo. TST003 is a first-in-class Gremlin1 targeting humanised monoclonal antibody.
Pharmaceutical Technology
OCTOBER 14, 2022
Pharmaceutical companies Xcell Biosciences (Xcellbio) and aCGT Vector have partnered to expedite the development of cell and gene therapies. The two companies will aim to improve the manufacturing and analytic procedures used to develop personalised cell and gene therapies to treat cancer patients.

pharmaphorum
FEBRUARY 17, 2025
Explore the latest advances in R&D and manufacturing of in vivo lentiviral vectors. Discover the challenges faced in this process and the innovative solutions being developed in the field.

Pharmaceutical Technology
FEBRUARY 13, 2023
The continued high level of interest in small molecules presents multiple opportunities to select a candidate that is ‘developable’, with subsequent rapid progression toward first-in-human (FIH) clinical testing. Therefore, it is vital to choose molecules for pharmaceutical development very carefully.

Pharmaceutical Technology
SEPTEMBER 19, 2022
The latest development is based on findings from the single-arm, open-label Phase II/III ALD-102 (Starbeam) and Phase III ALD-104 clinical trials. A one-time gene therapy, Skysona leverages ex-vivo transduction with the Lenti-D lentiviral vector for adding the ABCD1 gene’s functional copies into the hematopoietic stem cells of the patient.

BioSpace
OCTOBER 21, 2021
The experimental drug, NTLA-2001, is being developed for the treatment of transthyretin (ATTR) amyloidosis.
Pharmaceutical Technology
APRIL 28, 2023
Earlier in the year, the company had announced a move towards developing treatments for hemoglobinopathies like sickle cell disease and beta thalassemia and a focus on in vivo discovery. The US agency previously granted the Orphan Drug Designation to EDIT-301 for its study in beta thalassemia, in May 2022. g/dL and 45.4%
Pharmaceutical Technology
APRIL 4, 2023
Formerly known as CTX001, exa-cel is an investigational, autologous, ex vivo CRISPR/Cas9 gene-edited therapy. Vertex Pharmaceuticals chief medical officer and Global Medicines Development and Medical Affairs executive vice-president Carmen Bozic said: “The completion of our exa-cel global regulatory filings is a historic milestone.

Pharma Mirror
FEBRUARY 18, 2021
Maculus plans to raise USD3 million on a USD8 million valuation for a 12-18 month period, including initial in-vivo animal studies and completion of in-vitro / in-vivo drug elution studies. Maculus achieved the breakthrough using a patented novel tunable biodegradable proprietary product, MacuBloc.
BioTech 365
NOVEMBER 9, 2020
LIfT BioSciences N-LIfT Cell Therapy Successfully Infiltrates Pancreatic Tumours In-vivo LIfT BioSciences N-LIfT Cell Therapy Successfully Infiltrates Pancreatic Tumours In-vivo LIfT BioSciences N-LIfT Cell Therapy Successfully Infiltrates Pancreatic Tumours In-vivo London, 9 November 2020 –LIfT BioSciences, a biotech company developing (..)
Pharmaceutical Technology
AUGUST 18, 2022
An ex-vivo lentiviral vector (LVV) gene therapy, Zynteglo’s every dose is made by genetically modifying the bone marrow stem cells of the patient to produce functional beta-globin. thalassemia in adult and paediatric patients. Cell & Gene Therapy coverage on Pharmaceutical Technology is supported by Cytiva.

Pharmaceutical Technology
MARCH 29, 2023
Additionally, Eli Lilly is also developing orforglipron, purported to be the first triple-agonist therapy. In vivo data to date suggests therapeutic plasma levels of VK2735 may be achieved via oral dosing. The company hopes for results to be available in the second half of 2023. Following the announcement, Brian Lian, Ph.D,

Pharmaceutical Technology
FEBRUARY 23, 2023
Moderna has entered a strategic research and development partnership with ElevateBio’s Life Edit Therapeutics to discover and develop new in-vivo mRNA gene editing therapies. Moderna will also assume further development, manufacturing, and commercialisation responsibilities on exercising a target option.

Pharmaceutical Technology
OCTOBER 5, 2022
Patients with end-stage CLL have yet to benefit from an approved cell therapy, despite many developers initially trialling autologous CAR-T agents such as Yescarta and Kymriah in CLL during Phase I studies. Although CLL is a B-cell malignancy, patients also suffer from dysfunctional T-cells that exhibit an exhausted phenotype.

Pharmaceutical Technology
MAY 17, 2023
Scribe Therapeutics has entered a strategic collaboration with Eli Lilly and Company subsidiary Prevail Therapeutics for accelerating in vivo CRISPR-based therapies to target the causes of serious neurological and neuromuscular diseases. The company is also eligible for development and commercial milestone payments exceeding $1.5bn.

pharmaphorum
FEBRUARY 1, 2024
A single dose of a gene-editing drug being developed by Intellia Therapeutics almost eliminated attacks in patients with hereditary angioedema (HAE) in a clinical trial, pointing to the potential of CRISPR-based drugs delivered in vivo to treat diseases.

XTalks
NOVEMBER 25, 2024
XTALKS WEBINAR: Enhance Early Biomarker Discovery: Translating In Vitro and In Vivo Multi-Omics Oncology Data Live and On-Demand: Thursday, January 16, 2025, at 11am EST (4pm GMT/UK) Register for this free webinar to learn how to harness the full potential of multi-omics data and advanced patient models to drive early biomarker discovery.
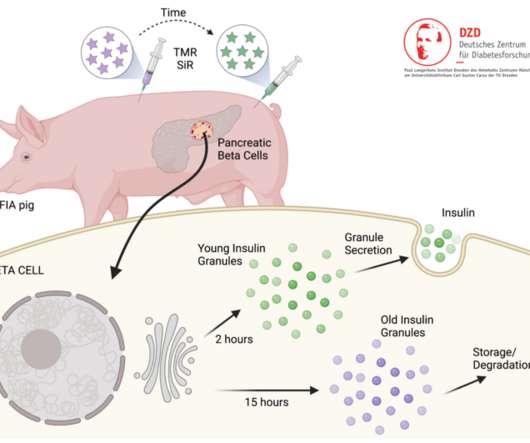
Scienmag
SEPTEMBER 29, 2021
Furthermore, the current understanding of beta cell function is mostly derived from studies of ex vivo isolated islets in rodent […]. However, the highly regulated process of insulin secretion, especially its molecular features and the stimuli behind this process have not yet been fully understood.
BioPharma Reporter
JUNE 13, 2023
Sygnature Discovery has launched an in vivo inflammation model, that the company says is âexciting but only the beginningâ of understanding life-limiting neuroinflammatory conditions.

Pharmaceutical Technology
MAY 12, 2023
The in vivo study, conducted in mice, involved a phosphatidylserine-containing nano-formulation of mRNA supplied by BioNTech, distinct from traditional lipid nanocrystals (LNCs). Matinas said it developed this unique formulation to “handle the physical complexity and biological fragility of mRNA”.

BioPharma Reporter
NOVEMBER 10, 2022
EdiGene Biotechnology USA has moved into a new Research & Development Center in Waltham, Mass. to advance its proprietary LEAPER tech into in vivo RNA editing therapies: with an initial focus on ophthalmology and the central nervous system.

XTalks
OCTOBER 20, 2023
Intellia said NTLA-2001 is the first investigational in vivo CRISPR-based gene editing therapy cleared to enter late-stage clinical development. As an in vivo therapy, it can edit genes inside the body rather than in cells extracted from patients. ATTR amyloidosis is a rare, progressive and fatal disease.

BioPharma Reporter
JANUARY 10, 2022
have announced a strategic collaboration and option agreement for the use of Mammothâs CRISPR systems to develop in vivo gene-editing therapies. Bayer AG and Mammoth Biosciences, Inc.,
Pharmaceutical Technology
AUGUST 21, 2022
-D-N4-hydroxycytidine (NHC) in vivo. Currently, the company is working with various regulatory agencies to explore the potential to further advance ASC10’s clinical development. A 240mg/kg twice a day dose of ASC10 led to a 4.0 A 240mg/kg twice a day dose of ASC10 led to a 4.0
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?


Let's personalize your content