Clearmind licenses psychedelic compounds for mental disorders
Pharmaceutical Technology
APRIL 18, 2024
Clearmind Medicine has entered into a licensing agreement with Yissum to develop Generation 3.0 psychedelic compounds for mental disorders.
This site uses cookies to improve your experience. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country, we will assume you are from the United States. Select your Cookie Settings or view our Privacy Policy and Terms of Use.
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.
Used for the proper function of the website
Used for monitoring website traffic and interactions
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.

Pharmaceutical Technology
APRIL 18, 2024
Clearmind Medicine has entered into a licensing agreement with Yissum to develop Generation 3.0 psychedelic compounds for mental disorders.

Bio Pharma Dive
AUGUST 2, 2022
Founded by serial entrepreneur Alexis Borisy, the company comes equipped with two experimental medicines it licensed from Merck KGaA and Blueprint Medicines.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Bio Pharma Dive
OCTOBER 22, 2024
The gene editing company will focus on “in vivo” medicines, while seeking to license out or find a development partner for its clinical-stage treatment reni-cel.
Drug Patent Watch
DECEMBER 12, 2024
Identifying branded drugs with a low likelihood of generic entry has become a crucial strategy for companies looking to expand their product portfolio through in-licensing. In this comprehensive guide, we’ll explore the intricacies of identifying such drugs and leveraging them for successful in-licensing opportunities.

Bio Pharma Dive
OCTOBER 9, 2024
The latest deal in AI drug discovery is a twist on the usual big pharma-startup collaboration model, with Insitro licensing technology and Lilly eligible for royalties.

Bio Pharma Dive
OCTOBER 27, 2020
The startup, which aims to develop lower-cost alternatives to branded medicines, has licensed two drugs from CStone Pharmaceuticals in a deal that could signal a coming price war in cancer immunotherapy.
Pharmaceutical Technology
APRIL 3, 2023
Ablaze Pharmaceuticals is set to develop a new GPC3-targeted peptide drug candidate for the treatment of liver cancer in China. The company is licensing the first-in-class drug candidate under an existing deal with RayzeBio. The agreement allows Ablaze to clinically develop and commercialise the drug in Greater China.

Pharmaceutical Technology
MARCH 24, 2023
Moderna has entered a strategic partnership with Generation Bio for the development of non-viral genetic medicines. Moderna’s biological and technical expertise will be combined with core technologies of the non-viral genetic medicine platform from Generation Bio.

Pharmaceutical Technology
NOVEMBER 15, 2024
MSD has secured an exclusive worldwide license from LaNova Medicines for developing, manufacturing, and commercialising the latter’s new investigational programmed cell death 1 (PD-1)/vascular endothelial growth factor (VEGF) bispecific antibody, LM-299.
Pharmaceutical Technology
JANUARY 5, 2023
Capsida Biotherapeutics and Eli Lilly and Company ’s wholly owned subsidiary Prevail Therapeutics have announced a partnership for the development of non-invasive gene therapies for central nervous system (CNS) diseases. The post Capsida Biotherapeutics and Prevail to develop CNS gene therapies appeared first on Pharmaceutical Technology.

Pharmaceutical Technology
FEBRUARY 3, 2023
The field of genomic medicine has reached a true turning point. With scientists fervently developing mRNA vaccines, nucleic acid therapeutics, and viral vector-based gene therapies, clinicians are set to have a growing number of tools available to treat a wide range of conditions, from infectious diseases to genetic disorders and more.

Pharmaceutical Technology
FEBRUARY 9, 2023
ReviR Therapeutics has signed a research collaboration and option-to-license agreement with Asieris Pharmaceuticals to discover new oncology therapeutics. ReviR combines computational and high throughput drug discovery technologies to deliver advanced medicines to patients.

Camargo
NOVEMBER 11, 2020
Each month, Camargo’s “In the News” series highlights important changes and advancements in the regulatory and development space and explores how those changes could impact your program. Unpacking the (Black) Box: Antares Licenses Urology Product with Boxed Warning. for patients with NTRK fusion cancer across all solid tumors.

Bio Pharma Dive
JUNE 21, 2024
A medicine Jazz acquired in 2019 missed the goal of a Phase 2 study. Elsewhere, Vanda rejected two takeover bids and Ashibio emerged from stealth with an antibody licensed from Gilead.
Pharmaceutical Technology
AUGUST 11, 2022
Gemini Therapeutics has signed a definitive agreement to merge with Disc Medicine in an all-stock deal to create a clinical-stage biopharmaceutical company. The merged company is expected to be named Disc Medicine, which will have corporate headquarters in Watertown, Massachusetts, US. Last year, Disc in-licensed bitopertin from Roche.
Pharmaceutical Technology
OCTOBER 4, 2022
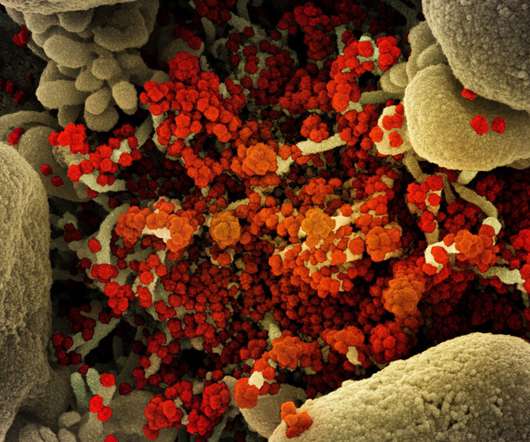
Shionogi & Co and the United Nations (UN)-backed public health organisation Medicines Patent Pool (MPP) have entered a voluntary licence agreement for the former’s oral Covid-19 antiviral candidate ensitrelvir fumaric acid (S-217622). An investigational Covid-19 drug, ensitrelvir is an inhibitor of 3CL protease.
Pharmaceutical Technology
JULY 28, 2022
Rezafungin is a new, once-weekly echinocandin antifungal being developed by Cidara to treat and prevent serious fungal infections such as candidemia and invasive candidiasis in adults. It is also being developed for invasive fungal infection prophylaxis in adults who undergo allogeneic blood and marrow transplantation. “By

Pharma Mirror
APRIL 18, 2023
Dudley, UK, April 18th 2023: Sterling Pharma Solutions, a global contract development and manufacturing organisation, today announced that it has been granted a Manufacturer’s Authorisation for Investigational Medicinal Products from the United Kingdom’s Medicines and Healthcare products Regulatory Agency (MHRA).
Outsourcing Pharma
SEPTEMBER 14, 2023
Insilico Medicine is due to receive $80 million upfront plus potential milestone payments as Exelixis gains global rights to develop and commercialize the Hong Kong firmâs small molecule cancer treatment.
Pharmaceutical Technology
JANUARY 23, 2023
Takeda has signed an exclusive licence agreement with HUTCHMED (China) and its subsidiary HUTCHMED to develop and market the latter’s fruquintinib. We look forward to utilising our development and commercial capabilities to expand the potential of this innovative medicine to patients beyond China. “We
Pharmaceutical Technology
APRIL 25, 2023
German biotechnology firm 3B Pharmaceuticals (3BP) has entered into a licensing agreement with Novartis Innovative Therapies for its fibroblast activation protein (FAP)-targeting peptide technology. 3BP receives an initial payment of $40m, and $425m as development, regulatory and commercial milestone payments.
Pharmaceutical Technology
APRIL 7, 2025
ABL Bio has announced a global licensing agreement with GSK, allowing the latter to develop medicines for neurodegenerative conditions.

Pharmaceutical Technology
JULY 22, 2022
Sangamo Therapeutics has received orphan medicinal product designation (OMPD) from the European Commission (EC) for its Investigational autologous Chimeric Antigen Receptor Regulatory T Cell (CAR-Treg) cell therapy, TX200, for solid organ transplantation.
Pharmaceutical Technology
DECEMBER 26, 2022
LegoChem Biosciences and Amgen have signed a multi-target research collaboration and license agreement to develop antibody-drug conjugates (ADC). As per the terms of the agreement, LegoChem will receive up to $1.25bn in upfront, development and commercial milestone payments from Amgen.

Bio Pharma Dive
DECEMBER 18, 2024
News of Merck’s licensing of Hansoh’s preclinical medicine pressured shares in Viking and other obesity drug developers seen as likely buyout targets.
Pharmaceutical Technology
JUNE 19, 2023
Bio-Thera Solutions and Biomm have entered a licensing and supply agreement for Bio-Thera’s BAT2206, a ustekinumab biosimilar. Bio-Thera will handle BAT2206’s global development and commercial supply out of its manufacturing plants in Guangzhou, China.

Pharmaceutical Technology
MAY 8, 2023
Shanghai Junshi Biosciences and Dr. Reddy’s Laboratories have partnered for the development and commercialisation of the anti-PD-1 monoclonal antibody, toripalimab, in 21 countries. The company may also choose to expand the scope to license toripalimab in New Zealand, Australia, and in nine other countries.

Pharmaceutical Technology
JUNE 23, 2022
It was discovered by Incyte and licensed to Novartis in 2009. The latest development comes after the European Medicines Agency’s (EMA) Committee for Medicinal Products for Human Use (CHMP) provided a positive opinion in April this year. A kinase inhibitor, Tabrecta acts on MET.

Pharmaceutical Technology
FEBRUARY 6, 2023
South Korean biotechnology company Qurient has entered a licence agreement with TB Alliance for the development and commercialisation of telacebec (Q203). Under the deal, TB Alliance will have the exclusive global license to develop and market telacebec except in Russia, South Korea, and the Commonwealth of Independent States (CIS) countries.
Pharmaceutical Technology
AUGUST 4, 2022
Poseida Therapeutics and Roche have signed a strategic partnership and licence agreement to develop allogeneic CAR-T cell therapies for hematologic malignancies. Roche and Poseida will also partner in a research programme for creating and developing next-generation features and enhancements for allogeneic CAR-T therapies.

Pharmaceutical Technology
APRIL 26, 2023
MiNA Therapeutics has entered into a research collaboration and option licensing agreement with BioMarin Pharmaceutical to speed up the development of therapeutic ribonucleic acid activation (RNAa) candidates to treat rare genetic diseases. The option licensing agreement is based on early-stage clinical results.
Pharmaceutical Technology
AUGUST 24, 2022
Lacerta Therapeutics has entered a new licensing and research partnership agreement with Eli Lilly and Company subsidiary, Prevail Therapeutics, to discover and develop adeno-associated virus (AAV) capsids for treating central nervous system (CNS) diseases. . Topic sponsors are not involved in the creation of editorial content.

Camargo
DECEMBER 13, 2021
The development of biological products (or biologics) represents a major advancement in modern medicine, enabling the treatment of patients with many illnesses where no other therapeutics were previously available. Regulatory Considerations for Biologics. BLA process (CBER). 510(k) process (CBER). NDA process (CBER).

Pharmaceutical Technology
OCTOBER 18, 2022
Gilead Sciences has entered an exclusive option and partnership agreement with MacroGenics for developing bispecific antibodies. Under the deal, the companies will leverage MacroGenics’ DART platform to develop MGD024 as well as two further bispecific research programmes.
Drug Patent Watch
OCTOBER 12, 2021
Imtiaz Hasan et al in Journal of Biosciences and Medicines under a Creative Commons Attribution 4.0 International License Abstract Development of generic drug product…. This paper was originally published by Md.

Pharmaceutical Technology
APRIL 27, 2023
The European Commission’s (EC) long-anticipated pharma reform plans in the European Union have finally been unveiled , indicating a focus on improving access to medicines across the bloc while cutting down on market exclusivity. However, the reform has its share of critics. Applications will now be digitised.

Bio Pharma Dive
MAY 30, 2023
The biotech, which went public in 2021 to develop a cancer drug licensed from Daiichi Sankyo, may try to acquire a different tumor-targeting medicine to grow its pipeline.
Pharmaceutical Technology
NOVEMBER 18, 2022
Regeneron Pharmaceuticals has entered a collaboration and licensing agreement with CytomX Therapeutics for developing conditionally-activated bispecific cancer therapies. This collaboration will use Regeneron’s Veloci-Bi bispecific antibody development platform and CytomX's Probody therapeutic platform.
Pharmaceutical Technology
AUGUST 25, 2022
The approval is based on comprehensive data from the clinical development programme of Roctavian, including two-year findings from the international Phase III GENEr8-1 clinical trial. The company plans to resubmit a Biologics License Application (BLA) for Roctavian by the end of next month.

XTalks
DECEMBER 4, 2024
In the Asia-Pacific market, Jupiter has negotiated partnerships with companies such as Sichuan Kelun and Tianjin Pharmaceuticals, focusing on Jotrol’s integration into traditional Chinese medicine frameworks. International patents valid until 2036 further strengthen its global positioning.

pharmaphorum
MARCH 24, 2022
GlaxoSmithKline has thrown its financial and drug development weight behind LifeMine Therapeutics, a US startup that aims to find new therapeutics from fungi – widely regarded as an underexplored resource of biologically-active compounds. The post GSK partners LifeMine on fungi-derived medicines appeared first on.

Pharmaceutical Technology
MARCH 2, 2023
resulted primarily from the development of the US dollar and the Chinese renminbi. Additionally, due to the acquisition of Exelead, a biopharmaceutical contract development and manufacturing company, sales increased by 0.4%. The increase in sales was due to new medicines. Organically, the company generated an increase of 6.4%
Pharmaceutical Technology
OCTOBER 14, 2022
Pharmaceutical companies Xcell Biosciences (Xcellbio) and aCGT Vector have partnered to expedite the development of cell and gene therapies. The two companies will aim to improve the manufacturing and analytic procedures used to develop personalised cell and gene therapies to treat cancer patients.
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?



Let's personalize your content