Seattle institute lands $18m funding to develop RNA vaccine for chikungunya
Pharmaceutical Technology
AUGUST 3, 2023
AAHI is developing a novel RNA vaccine that overcomes manufacturing and storage limitations to increase accessibility.
This site uses cookies to improve your experience. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country, we will assume you are from the United States. Select your Cookie Settings or view our Privacy Policy and Terms of Use.
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.
Used for the proper function of the website
Used for monitoring website traffic and interactions
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.

Pharmaceutical Technology
AUGUST 3, 2023
AAHI is developing a novel RNA vaccine that overcomes manufacturing and storage limitations to increase accessibility.
Pharmaceutical Technology
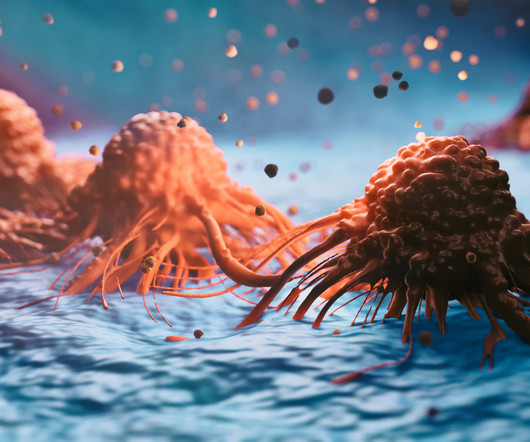
MAY 11, 2023
Results published in Nature for a personalised pancreatic cancer vaccine that uses neoantigens from patients’ tumours have lent further support to early positive signals. The vaccine, developed by BioNTech, led to half of the patients with pancreatic cancer in the Phase I trial remaining cancer-free 18 months later.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

World of DTC Marketing
DECEMBER 29, 2020
OBSERVATION: Biologics can take a long time to develop but COVID vaccines have been in development for almost 50 years and novel approaches were used to develop these vaccines. Vaccines typically take 10 to 15 years to develop, test and release to the public. However, this is time-consuming.

Pharmaceutical Technology
MARCH 28, 2023
Following on from its Covid-19 vaccine programmes, BioNTech has set its sights on a range of infectious diseases for vaccine development. The company saw major successes with its Covid-19 vaccine, developed in collaboration with Pfizer. In response to the lower vaccine sales forecasts, BioNTech shares opened at 3.9%
Pharmaceutical Technology
JUNE 1, 2023
A new intracellular drug delivery centre will be established in the UK to support potential ribonucleic acid (RNA) vaccines and therapeutics , as well as the development of innovative drug delivery technologies. It will focus on studying and developing new lipid nanoparticle (LNP) formulations for the delivery of RNA medicine.
Pharmaceutical Technology
OCTOBER 14, 2022
German pharmaceutical firm Merck has extended its partnership with Moderna to jointly develop and sell mRNA-4157/V940, an investigational personalised cancer vaccine (PCV). In 2016, the companies entered a strategic partnership to develop novel messenger RNA (mRNA) based PCVs.

Pharmaceutical Technology
AUGUST 1, 2022
GreenLight Biosciences has entered a partnership with the US National Institutes of Health (NIH) for the development of Covid-19 vaccines, which offer broader protection against new variants and with durable effects. They intend to develop vaccines that provide lasting immune responses compared to existing vaccines.

AuroBlog - Aurous Healthcare Clinical Trials blog
OCTOBER 16, 2022
The husband-and-wife team who co-founded BioNTech, the biotechnology company that partnered with Pfizer to develop an effective messenger-RNA (mRNA) shot against COVID-19, has predicted that a cancer vaccine could be widely available within the next decade.

Pharmaceutical Technology
APRIL 16, 2024
Sanofi has signed an agreement with IDT Australia for preclinical formulation development of its messenger RNA (mRNA) vaccines.
Pharmaceutical Technology
DECEMBER 11, 2022
The US Food and Drug Administration (FDA) has granted Fast Track designation for Pfizer and BioNTech’s messenger ribonucleic acid (mRNA)-based combination vaccine candidate against Covid-19 and influenza. The vaccine is intended to prevent two respiratory ailments through a single injection. 5 Omicron sublineages spike proteins.
Pharmaceutical Technology
AUGUST 1, 2022
Moderna has entered a new supply contract with the US Government to deliver 66 million doses of its Covid-19 vaccine booster candidate, mRNA-1273.222. The contract comprises a $1.74bn award to produce and supply these vaccine doses and options to further procure up to 234 million additional doses of the company’s booster candidates.

Pharmaceutical Technology
AUGUST 17, 2022
Merck (MSD outside North America) has entered a partnership agreement with Orna Therapeutics for discovering, developing and marketing various programmes based on next-generation RNA technology. These programmes will include therapies and vaccines in infectious disease and oncology areas.
Pharmaceutical Technology
FEBRUARY 3, 2023
Only a few weeks into the new year, the prospect of getting a successful advanced HIV vaccine shrank after the discontinuation of yet another late-stage trial. On January 18, Janssen, a Johnson & Johnson (J&J) subsidiary, stated that its vaccine was not effective in preventing HIV infections.
Pharmaceutical Technology
MAY 2, 2023
4/5 Covid-19 messenger RNA (mRNA) vaccines. The expanded EUAs state that these current bivalent vaccines are now to be used for all primary and booster doses administered to individuals ages six months of age and older. 1 vaccines by both companies were revoked by the FDA in August 2022, after the BA.5

Pharmaceutical Technology
AUGUST 2, 2022
Samsung Biologics and GreenLight Biosciences have completed the initial commercial-scale engineering run for their messenger ribonucleic acid (mRNA) Covid-19 vaccine under their manufacturing collaboration. Following the demonstration at Samsung, the clinical trial of GreenLight’s Covid-19 booster vaccine is anticipated to commence this year.
Pharmaceutical Technology
MARCH 1, 2023
The Covid-19 pandemic led to massive developments and scientific advances within the field of messenger ribonucleic acid (mRNA) vaccines. Pfizer /BioNTech and Moderna’s mRNA-based vaccines were the first to receive emergency use authorisation (EUA) out of all other Covid-19 vaccines under development in late 2020.

Pharmaceutical Technology
MAY 15, 2024
CircRNA is still in early days of development, but could be in trials as vaccines, therapeutics and biomarkers in the next few years.
STAT News
NOVEMBER 24, 2022
An experimental influenza vaccine developed using messenger RNA technology appears capable of inducing what should be a protective immune response against all known subtypes of flu, at least in animals. If the work is translated into humans it could turn out to be a version of a long-sought universal vaccine.
Pharmaceutical Technology
NOVEMBER 30, 2022
in a project agreement from the US government for developing self-amplifying RNA (saRNA) vaccine technology against advanced and emergent viral threats. Development of vaccines to Phase I trials under the five-year $59m prototype project comprises additional $28.4m in milestone payments.

Pharmaceutical Technology
NOVEMBER 2, 2023
While things may have slowed down a little since the rapid approvals of the COVID-19 vaccines, the excitement around RNA-LNP is still going strong.

Bio Pharma Dive
OCTOBER 6, 2022
A new agreement with the Australian government will bring messenger RNA research and manufacturing capabilities to the country, adding to the COVID-19 vaccine developer’s global expansion efforts.

pharmaphorum
MAY 2, 2024
A personalised vaccine for the aggressive brain cancer glioblastoma developed has shown encouraging signs of efficacy in its first human trial.
Pharmaceutical Technology
APRIL 27, 2023
Orbital Therapeutics has raised $270m in a Series A round led by ARCH Venture Partners to advance a portfolio of programmable RNA therapeutics. Orbital will use the new funding to increase the application of RNA-based medicines for use in the fields of new vaccines, immunomodulation and protein replacement.

Pharma Mirror
MARCH 29, 2022
NIEL, BELGIUM, eTheRNA Manufacturing, a specialist RNA process developer and manufacturing member of the Belgian eTheRNA group, is introducing a new Lipid Nanoparticle (LNP) formulation development and production service to support the discovery and early pre-clinical development of RNA-based therapeutics and vaccines.
BioSpace
APRIL 11, 2021
Researchers are leveraging the messenger RNA (mRNA) technology used to develop the Pfizer-BioNTech and Moderna COVID-19 vaccines for possible treatments for a range of other diseases, including HIV and cancer.
Pharmaceutical Technology
JUNE 30, 2022
HDT Bio has received Emergency Use Approval from Indian regulators for its Covid-19 vaccine, Gemcovac. The vaccine leverages self-amplifying RNA (saRNA), which can replicate itself after administration and could be effective at very low doses. HDT Bio CEO Steve Reed said: “Our saRNA vaccine is a game-changer.

XTalks
SEPTEMBER 13, 2021
With RNA therapies being the next hot thing in genetic medicine, Eli Lilly is joining the RNA editing race by partnering with Netherlands-based ProQR Therapeutics NV (Nasdaq: PRQR), a biotech company developing RNA-based therapies for rare genetic diseases with a focus on blinding disorders of the retina.

Scienmag
JULY 6, 2022
Army scientists and industry partners were among the first to demonstrate that messenger RNA (mRNA)—the technology recently used in COVID-19 vaccines and others—could also be used to develop treatments for infectious diseases. Credit: Study: USAMRIID / […].
Pharmaceutical Technology
APRIL 20, 2023
Targovax has unveiled plans to rebrand as Circio , reflecting its strategic shift to focus on expediting the development of its innovative circular RNA (circRNA) platform. Initially reported in 2011, CircRNA is a naturally occurring class of RNA.

pharmaphorum
AUGUST 16, 2022
Merck & Co has ramped up its involvement in the RNA category, partnering with US biotech Orna Therapeutics in a deal valued at up to $3.5 billion, consummating a relationship that first started in 2018 and spanned a range of mRNA-based vaccines for infectious diseases. billion, including $150 million upfront.
Pharmaceutical Technology
SEPTEMBER 1, 2022
The US Food and Drug Administration (FDA) has granted Emergency Use Authorizations (EUAs) for bivalent formulations of Moderna and Pfizer -BioNTech’s Covid-19 vaccines as boosters. According to the amended EUA, the vaccines are indicated to be administered at a minimum of two months after the initial or booster dose.

XTalks
NOVEMBER 9, 2021
Perhaps popularized by the COVID-19 vaccines, RNA-based technologies now have the potential to become the next best pesticide to combat crop pests, like insects and fungal pathogens. Over the last year, researchers have been studying the effectiveness of RNA-based pesticides, and there are already a handful of sprays in the works.

XTalks
MARCH 21, 2022
Credited as a pioneering RNA interference (RNAi) therapeutics company, Alnylam Pharmaceuticals is taking Pfizer and Moderna to court, claiming that the companies’ use of the lipid nanoparticle (LNP)-based RNA delivery technology in their mRNA COVID-19 vaccines infringes on a patented technology.
Pharmaceutical Technology
FEBRUARY 15, 2023
The swift development and deployment of messenger-RNA (mRNA) vaccines against the SARS-CoV-2 virus during the COVID-19 crisis has catapulted the pharmaceuticals industry into a new paradigm. Used in the COVID-19 vaccines, LNPs have shown a promising track record of protecting the mRNA cargo from degradation.

Pharmaceutical Technology
FEBRUARY 1, 2023
RSV researchers at major pharmaceutical companies are currently working to develop new RSV drugs to beat future waves of RSV infection and gain the first RSV vaccine FDA approval. Pharmaceutical companies are pushing to develop drugs and vaccines for RSV with these populations in mind.

pharmaphorum
MARCH 16, 2021
European regulators questioned the integrity of early batches of Pfizer/BioNTech’s mRNA vaccine, although the matter was resolved before approval, according to information leaked online following a cyberattack. As it conducted its analysis of the vaccine in December, the European Medicines Agency’s systems were targeted by unknown hackers.

Pharmaceutical Technology
JULY 18, 2022
Moderna has received provisional registration from the Australian Therapeutic Goods Administration (TGA) for its messenger RNA (mRNA) Covid-19 vaccine, Spikevax, for kids aged six months to five years. The 25µg two-dose vaccine regimen is indicated for active immunisation for the prevention of Covid-19.
Pharmaceutical Technology
AUGUST 24, 2022
Mode rna has submitted an application to the US Food and Drug Administration (FDA) to obtain emergency use authorization (EUA) for mRNA-1273.222, its BA.4/BA.5 5 Omicron-targeting bivalent booster vaccine for Covid-19. The submission is made for a 50µg booster dose of the vaccine for usage in adults aged 18 years and above.

STAT News
JANUARY 19, 2023
And do normal people care about messenger RNA? Journalist Nathan Vardi joins us to talk about his new book delving into the race to develop the lifesaving cancer drug now called Imbruvica, involving a Scientologist CEO and secretive investor seeking redemption after the worst trade of his life. Where do new drugs come from?
pharmaphorum
MAY 11, 2021
Flagship Pioneering, the VC fund run by Moderna’s co-founder Noubar Afeyan has launched a new biotech Laronde , with an ambitious plan to create a new class of drugs based on Endless RNA. The post Moderna’s founder launches Laronde, promising new ‘Endless RNA’ drug class appeared first on.

World of DTC Marketing
APRIL 15, 2021
QUICK READ: Some deaths will occur during the COVID-19 vaccination rollout, but these deaths would have happened for other reasons and are unrelated to the vaccine. In some instances, vaccine-hesitant activists are manufacturing stories of deaths related to the vaccine that never happened.
Pharmaceutical Technology
FEBRUARY 15, 2023
In the last three years alone, there have been over 633,000 patents filed and granted in the pharmaceutical industry, according to GlobalData’s report on Innovation in Pharmaceuticals: Flavivirus vaccine components. Sanofi is one of the leading patent filers for Flavivirus vaccine components.
Pharmaceutical Technology
MARCH 23, 2023
China’s National Medical Products Administration (NMPA) has granted emergency use authorisation (EUA) for CSPC Pharmaceutical Group’s messenger RNA (mRNA) vaccine, SYS6006, to treat Covid-19. The independently developed SYS6006 vaccine has been designed to target some major Omicron variants. efficacy after 14 to 28 days.

Pharmaceutical Technology
MAY 8, 2023
Within the emerging innovation stage, cell therapy for ocular disorders, coronavirus vaccine components, and DNA polymerase compositions are disruptive technologies that are in the early stages of application and should be tracked closely. These viruses carry the genetic information to synthesise an RNA-dependent RNA polymerase.
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?


Let's personalize your content