The Utility of Liquid Biopsy in Oncology Clinical Trials
XTalks
NOVEMBER 7, 2022
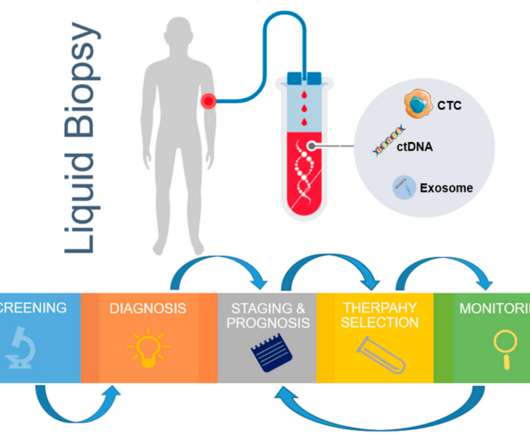
Liquid biopsy tests in oncology involve isolating entities such as circulating tumor cells (CTC), circulating tumor DNA (ctDNA) and tumor-derived exosomes. The cfDNA that originates from tumors is called circulating tumor DNA (ctDNA). These tumor-derived entities are used to derive genomic and proteomic data.









Let's personalize your content