CPHI Europe: Gummies as a drug delivery system could improve compliance
Pharmaceutical Technology
OCTOBER 10, 2024
At CPHI Europe, an expert discussed the promise of gummies as a drug delivery system and their unique manufacturing challenges.
This site uses cookies to improve your experience. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country, we will assume you are from the United States. Select your Cookie Settings or view our Privacy Policy and Terms of Use.
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.
Used for the proper function of the website
Used for monitoring website traffic and interactions
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.

Pharmaceutical Technology
OCTOBER 10, 2024
At CPHI Europe, an expert discussed the promise of gummies as a drug delivery system and their unique manufacturing challenges.
Roots Analysis
FEBRUARY 26, 2024
Biologics constitute a majority of the top selling drugs and represent one of the fastest growing segments of the overall pharmaceutical industry. In fact, the share of biologics in the overall pharmaceutical contract manufacturing market has increased from 16% in 2006 to over 25% in 2017.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Roots Analysis
MARCH 1, 2022
Interestingly, over the past few years, drug developers focused on non-respiratory diseases have also shifted their attention towards intranasal drug formulations. This can be attributed to ease of delivery, increased bioavailability and by-pass of first-pass metabolism offered by this type of route of administration.

Pharmaceutical Technology
OCTOBER 26, 2022
Nano-based delivery systems are on the rise, as they enable manufacturers to deliver therapeutic agents to specific targeted tissue in a more controlled manner. Data indicates that the global nanopharmaceutical drugs market size reached USD 53.85 Billion in 2021 and is expected to reach USD 102.4 Billion in 2030.
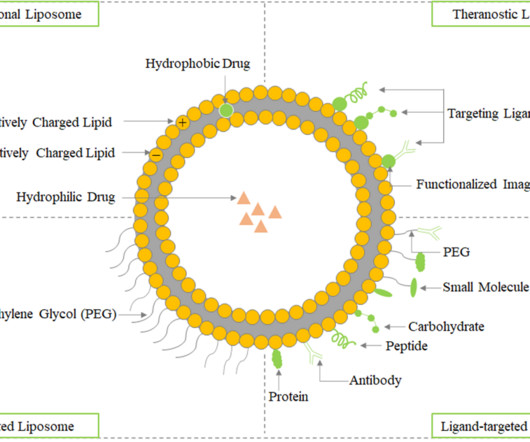
Roots Analysis
AUGUST 8, 2023
Furthermore, a wide variety of drugs and macromolecules, including DNA , proteins, and imaging agents, can be encapsulated in liposomal vesicles due to their unique ability to entrap both lipophilic and hydrophilic substances. The various therapeutic applications of liposomes in drug delivery have been highlighted in the figure.

Pharmacy Checkers
NOVEMBER 6, 2020
Importation of prescription drugs to help lower prices for Americans will remain a major issue no matter who is president next year. The more we know about where our meds are made (mostly not here) the less successful drug industry lobbying will be against lower-cost, imported medicines. Three countries have a hand in making it.
XTalks
JANUARY 29, 2025
Highlights include expansion of biotech operations in Philadelphia, acquisition of a new pharma-device facility near Dublin, Ireland, and a new Center of Excellence for advanced drug delivery and drug-device combination product assembly in Rockford, Illinois.
Pharmaceutical Technology
NOVEMBER 15, 2022
When it comes to vaccines and other high-value biologics, it is essential that effective formulations go hand in hand with safe delivery. By having the drug already dosed inside the chamber and no need to draw it from a vial into a syringe, the process is streamlined and the risk of a potential dosing error is eliminated.

Pharmaceutical Technology
FEBRUARY 27, 2023
According to GlobalData, Phase I drugs for Adrenal Insufficiency have a 67% phase transition success rate (PTSR) indication benchmark for progressing into Phase II. GlobalData’s report assesses how Hydrocortisone’s drug-specific PTSR and Likelihood of Approval (LoA) scores compare to the indication benchmarks.

Pharmaceutical Technology
SEPTEMBER 20, 2022
The information contained within the download document is intended for pharmaceutical manufacturers, wholesalers, retailers and distributors, pharmaceutical executives, medical representatives, business development managers, retail salesmen, sales managers, pharmacy executives, and any other individual involved in pharmaceutical marketing.

Pharmaceutical Technology
NOVEMBER 7, 2022
SHL Medical is a world-leading solutions provider for advanced drug delivery systems with a unified purpose to further enable patient independence. Currently, SHL Medical has four locations, in Switzerland, Taiwan, Sweden, and the US, with Taiwan being the main manufacturing location.
Roots Analysis
MARCH 13, 2022
Given the evident benefits of biologics over small molecule drugs (including high efficacy, target specificity and favorable safety profiles), the biopharmaceutical market is poised to witness continued and consistent growth over the next several years. As a result, the demand for biopharmaceutical excipients has grown considerably.

Roots Analysis
FEBRUARY 24, 2022
The overall manufacturing of oligonucleotides involves the following steps. Owing to the incessant demand for oligonucleotide-based products, several small to mid-sized companies and certain pharma giants, have begun outsourcing their manufacturing operations to contract service providers. Accelerating Oligonucleotide Demand.

Roots Analysis
FEBRUARY 18, 2022
The current landscape consists of more than 95 players that claim to offer oligonucleotide contract manufacturing services for research, diagnostic and therapeutic applications. It is worth highlighting that major share of the global oligonucleotide manufacturing capacity is installed in North America, primarily in the US. Web: [link].
Roots Analysis
FEBRUARY 26, 2023
Over the last two decades, the pharmaceutical industry has observed a paradigm shift from conventional drug delivery strategies to the long-acting drug delivery (LADD) of products to treat several disease indications, such as ophthalmic disorders, oncological disorders, neurological disorders and infectious diseases.
STAT News
NOVEMBER 3, 2022
The companies allege Moderna’s vaccine uses their technology for a drug-delivery system without permission. Citing Lilly announced earlier this year that the creation of its proposed manufacturing campus would create 300 permanent jobs once operational as well as 500 jobs during the construction phase.
Roots Analysis
MARCH 21, 2022
However, several drug delivery devices that enable patients to self-administer their respective medications are now available. Subcutaneous drug delivery systems – the helping hand for patients. Examples of some of the most popular types of drug delivery systems have been depicted below.

XTalks
MAY 30, 2023
The healthcare industry has one of the heaviest environmental footprints, and manufacturers are increasingly faced with regulations to make more sustainable products,” said Mohammad H. The patch itself is made of biodegradable nanocellulose and contains no plastic additives. Behfar, senior scientist at VTT, in the news release.

BioTech 365
AUGUST 4, 2020
Advancing toward commercialization as base material for drug delivery systems TOKYO–(BUSINESS WIRE)–Asahi Kasei has established industrial manufacturing technology for hyaluronic acid (HA) nanogel* as a new pharmaceutical excipient, and is providing samples to prospective customers in order to commercialize the … Continue reading (..)

Roots Analysis
MARCH 16, 2022
In order to promote the use of exosomes in various therapeutic applications including targeted drug delivery vehicles, engineered exosomes and many more, there are a number of companies that offer services related to the isolation, purification, characterization or quantification of exosomes. Want additional details on the pipeline?

Roots Analysis
MARCH 2, 2022
However, it is important to highlight that there are still several challenges which need to be addressed in order to develop biologic drugs capable of effectively being administered via the oral route without undergoing significant losses in specificity and / or bioavailability. Oral Protein / Peptide-based Drugs. Web: [link].

FDA Law Blog
SEPTEMBER 19, 2023
The six-page statement explains that “Brand drug manufacturers may be harming generic competition through the improper listing of patents in the. Of course, the statute says that only drug formulation, composition, or method of use patents are listable, but FDA has not defined the scope of the “drug” that must be covered by the patent.

Roots Analysis
FEBRUARY 20, 2022
Over the last decade, one of the major challenges faced by pharmaceutical players across the globe is low drug solubility. As a matter of fact, recently many drug developers have initiated formulating novel therapeutic interventions that utilize nanoparticles as a major component ( nanomedicines ).
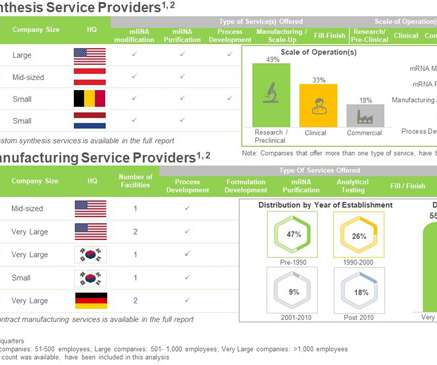
Roots Analysis
FEBRUARY 13, 2023
As a result, there is an evident increase in the demand for mRNA manufacturing capacity. The key applications of mRNAs are below: Contract Manufacturing in mRNA Synthesis AND Manufacturing Service Domain The synthesis and manufacturing process of mRNA-based therapeutics / vaccines is complex and associated with several challenges.
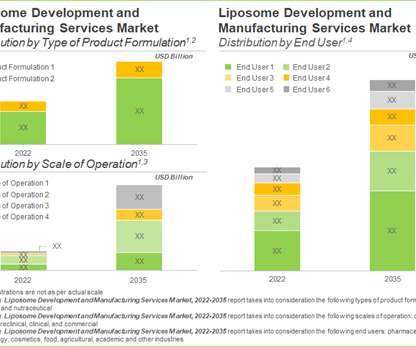
Roots Analysis
FEBRUARY 14, 2023
In recent years, liposomes have garnered significant interest of researchers, as well as industry players, owing to their potential in diagnosis and treatment of various diseases, with a focus on delivering drugs and genetic material.
The Pharma Data
SEPTEMBER 1, 2021
Louis have genetically engineered cells that, when implanted in mice, will deliver a biologic drug in response to inflammation. The approach allows those cells to remain in the body for a long time and secrete a drug whenever there is a flare of inflammation.” million adults in the United States.

Roots Analysis
MAY 21, 2024
Over the last decade, one of the major challenges faced by pharmaceutical companies across the globe is low drug solubility. In fact, it has been observed that around 40% of the pharmaceutical drugs approved by regulatory organizations exhibit poor bioavailability / solubility.

XTalks
AUGUST 24, 2022
The US Food and Drug Administration (FDA) also has concerns about the toxic effects of EtO and thus is continuing to encourage new ways to sterilize medical devices and strategies to mitigate the emission levels of EtO while achieving adequate sterilization.

Roots Analysis
FEBRUARY 16, 2022
In earlier times, container closure integrity testing was not regarded as an essential step in the process of manufacturing. The company developing drug product through such an intense process, use to consider container closure system only for packaging of the product. [1] Container Closure Integrity Testing Services Market.

Roots Analysis
FEBRUARY 26, 2024
However, certain biological targets have long eluded drug development efforts. Likewise, there are several other targets that have not yet been successfully drugged and researchers are making significant efforts to identify novel approaches to target them.

FDA Law Blog
MARCH 12, 2024
Koblitz — After years of silence from FDA on whether certain patents could be listed in the Orange Book, some manufacturers of drug and device combination products have had a rude awakening lately.

Roots Analysis
OCTOBER 5, 2023
Driven by the increasing need for safe and efficient medical devices and drug delivery systems, the pharmaceutical polymers / medical grade polymers domain is advancing significantly. There has been a rise in the development of polymers for biopharma with improved biocompatibility, biodegradability and functionality.
Roots Analysis
FEBRUARY 18, 2022
In the past decade, more than 1,500 clinical trials have been registered for evaluating drug therapies targeting myeloid cells. Roots Analysis – MYELOID CELL TARGETING THERAPEUTICS: BUDDING THERAPY AGAINST CANCER. CLINICAL TRIALS EVALUATING MYELOID CELLS TAREGTING THERAPEUTICS. More than 380,000 patients have been enrolled till date.
Roots Analysis
MARCH 3, 2022
Since the approval of Soliris® in 2007, an anti-C5 antibody, the field of complement drug discovery has gained significant attention. Currently, nine complement therapeutics are commercially available, while around 190 molecules are under development for various disease indications. Next Generation Complement Therapeutics Market. Web: [link].

FDA Law Blog
NOVEMBER 7, 2023
Mass hysteria! ” At least that’s this blogger’s reaction to the recent news that FTC sent out Notice letters to 10 different drug companies about the patent information they list in the Orange Book. Koblitz — “ Human sacrifice! Dogs and cats living together! And this is the first inkling we have about the government’s position.
Delveinsight
NOVEMBER 29, 2020
Therapies such as dexamethasone drug delivery system and vascular endothelial growth factor inhibitors have already pierced deeper into the market. Looking at its potential, the drug is also being watched out in diabetic macular edema in combination with intravitreal anti-VEGF drugs.

The Pharma Data
NOVEMBER 25, 2020
“Reliable testing and certified personal protective equipment manufactured in the United States plays a key role in the pandemic response and getting our communities back to work. Vivera is vertically integrated with patented technology, manufacturing capabilities, and distribution for its products. .

Roots Analysis
JANUARY 17, 2024
They offer several advantages over traditional drug delivery systems ( such as vials and syringes ), including reduced chances of dosing errors, increased patient compliance and decreased risk of microbial contamination.
Roots Analysis
MARCH 24, 2022
However, research related to metabolites is essential to increase the understanding of molecular level interactions, functions, modifications and regulations in cells, therefore, the field of metabolomics plays a vital role in biomarker discovery for early disease diagnosis and prognosis as well as for drug discovery and development processes.

Roots Analysis
SEPTEMBER 13, 2023
It is worth mentioning that the first radiopharmaceutical peptide-drug conjugate Luthathera was approved by United States Food and Drug Administration (USFDA) in 2018 for the treatment of gastroenteropancreatic neuroendocrine tumors. It consists of somatostatin-derived peptide and DOTA complexed with radioactive isotope 77 Lu.

Pharmaceutical Technology
MARCH 22, 2023
The demand for injectable drugs is rising. According to the GlobalData report Contract Injectable Packaging Trends in the Bio/Pharma Industry , more than half (55%) of FDA drug approvals in 2021 were accounted for by injectables. Several drug development trends are driving injectables demand.

Pharmaceutical Technology
FEBRUARY 3, 2023
In 2021, 72% of newly approved drugs were small molecules, and almost 50% of new drugs approved were OSD. While the industry is seeing advances in alternative drug delivery systems, oral solid doses, such as pills, capsules and soft gels, remain at the forefront of the industry.

Drug Patent Watch
MARCH 11, 2025
Unlocking the Path to Generic Drug Development: A Freedom to Operate Analysis As a generic drug manufacturer, navigating the complex landscape of biopharmaceutical development can be daunting. But what exactly is an FTO analysis, and why is it essential for your generic drug stability testing?

Drug Patent Watch
DECEMBER 19, 2024
A robust drug patent portfolio is not just an assetit’s a critical tool for success. This comprehensive guide will explore how pharmaceutical companies can leverage their drug patent portfolios to maximize value, maintain market dominance, and drive innovation.
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?


Let's personalize your content