FDA approves injectable antibiotics for B Braun drug delivery system
Pharmaceutical Technology
APRIL 10, 2025
The FDA has granted approval for the injectable antibiotic, Piperacillin and Tazobactam, to be used in B Braun's drug delivery system.
This site uses cookies to improve your experience. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country, we will assume you are from the United States. Select your Cookie Settings or view our Privacy Policy and Terms of Use.
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.
Used for the proper function of the website
Used for monitoring website traffic and interactions
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.

Pharmaceutical Technology
APRIL 10, 2025
The FDA has granted approval for the injectable antibiotic, Piperacillin and Tazobactam, to be used in B Braun's drug delivery system.
Pharmaceutical Technology
SEPTEMBER 16, 2024
Roche has secured approval from the US Food and Drug Administration for Ocrevus Zunovo with Halozyme's Enhanze drug delivery technology.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
Pharmaceutical Technology
FEBRUARY 14, 2023
In the last three years alone, there have been over 633,000 patents filed and granted in the pharmaceutical industry, according to GlobalData’s report on Key innovation: Leading companies in nanoparticles for drug delivery. Bristol-Myers Squibb is the leading patent filer in nanoparticles for drug delivery.

Pharma Mirror
FEBRUARY 18, 2021
Maculus plans to raise USD3 million on a USD8 million valuation for a 12-18 month period, including initial in-vivo animal studies and completion of in-vitro / in-vivo drug elution studies. Capable of delivering any FDA approved.

Pharmaceutical Technology
APRIL 24, 2023
Researchers may develop nanodrugs due to their smaller surface area to allows drugs to dissolve faster, or nanoparticles may encapsulate drugs, so they last longer in the body. Covid-19 vaccines from Moderna and Pfizer /BioNTech, which use lipid nanoparticles, became the only two FDA-approved vaccines for almost all ages.

Fierce Pharma
FEBRUARY 7, 2023
Orexo, leveraging drug delivery tech, seeks FDA approval of nasal high-dose opioid rescue medicine ntaylor Tue, 02/07/2023 - 08:18

XTalks
OCTOBER 27, 2021
Genentech’s ocular implant drug delivery system for treatment of macular degeneration has received approval from the US Food and Drug Administration (FDA). Susvimo delivers ranibizumab via a port delivery system into a surgically placed implant in the eye, allowing for continuous delivery of the drug.

Pharmaceutical Technology
APRIL 17, 2023
US-based development-stage biopharmaceutical firm Satsuma Pharmaceuticals is developing STS101, a unique nasal powder formulation of the anti-migraine drug dihydroergotamine mesylate, for the treatment of acute migraine. In March 2023, Satsuma submitted a new drug application for STS101 to the US Food and Drug Administration (FDA).

XTalks
JULY 26, 2024
These issues primarily stem from patients and healthcare providers miscalculating or incorrectly administering the drug. Compounded semaglutide products, unlike the US Food and Drug Administration (FDA)-approved ones, can vary widely in strength and packaging, making it easy for mistakes to happen.
XTalks
NOVEMBER 4, 2021
Now, instead of reaching for reading glasses, AbbVie’s eyedrop formulation Vuity could offer help in correcting far-sightedness as the US Food and Drug Administration (FDA) granted approval to it late last week. With the approval, Vuity becomes the first eyedrop approved for presbyopia. Vuity is a 1.25

Scienmag
JUNE 3, 2022
The repurposing of FDA-approved drugs for alternative diseases is a faster way of bringing new treatments into the clinic. Researchers at Karolinska Institutet in Sweden have repurposed a cancer drug for treatment of neuroinflammatory diseases such as multiple sclerosis.

Pharmaceutical Technology
FEBRUARY 13, 2023
Elyxyb is a prescription nonsteroidal anti-inflammatory drug (NSAID) used for acute migraine treatment in adult patients with or without aura. It is formulated using a self-micro-emulsifying drug delivery system that improves the drug’s bioavailability and solubility.

XTalks
MARCH 21, 2025
The rankings are based on each companys ability to tackle critical challenges from drug delivery and diagnostics to personalized therapies using cutting-edge science. Following Neffys FDA approval , ARS Pharma reported $2.3 Recursion Recursion launched the LOWE AI-driven drug discovery platform, reporting $51.1
Outsourcing Pharma
AUGUST 13, 2024
Patients suffering from severe allergic reactions now have access to a new, needle-free treatment option, as the US Food and Drug Administration (FDA) has approved neffy, a 2 mg epinephrine nasal spray developed by ARS Pharmaceuticals, Inc.

XTalks
AUGUST 14, 2024
The US Food and Drug Administration (FDA) has approved Galderma’s monoclonal antibody Nemluvio (nemolizumab) for the treatment of adult patients with the chronic skin condition prurigo nodularis. Monoclonal antibody therapy has been found to have fewer side effects when compared to immunosuppressant drugs.

XTalks
SEPTEMBER 16, 2024
In a new regulatory milestone, the US Food and Drug Administration (FDA) has approved Ocrevus Zunovo (ocrelizumab & hyaluronidase-ocsq), introducing the first-ever subcutaneous injection option for relapsing multiple sclerosis (RMS) and primary progressive multiple sclerosis (PPMS). billion in 2024 to $38.94

XTalks
JANUARY 22, 2025
Valneva SE, who developed the worlds first FDA-approved chikungunya vaccine , has now unveiled promising Phase III data for its single-shot chikungunya vaccine, IXCHIQ, in adolescents aged 12 to 17. Following the announcement, Valnevas stock rose by 1.4%.

XTalks
JUNE 14, 2023
percent received approval from the US Food and Drug Administration (FDA) to treat dry eye disease. Attendees will understand best practices for novel drug delivery design and development. Attendees will understand best practices for novel drug delivery design and development. What Is Dry Eye Disease?

Outsourcing Pharma
JULY 23, 2024
Rusan Pharma Private Limited has announced that its active pharmaceutical ingredient (API) manufacturing facility in Ankleshwar, Gujarat, has been granted Good Manufacturing Practice (GMP) approval by the FDA.

XTalks
JANUARY 17, 2023
The US Food and Drug Administration (FDA) granted approval to AstraZeneca’s Airsupra (albuterol/budesonide), formerly known as PT027, for the as-needed treatment or prevention of bronchoconstriction and to reduce the risk of asthma attacks in individuals aged 18 years and older who have asthma.

pharmaphorum
NOVEMBER 4, 2020
That includes include France’s Inotrem, which is developing nangibotide in phase 2 testing for septic shock, and Dublin-based Priothera, which is developing immuno-oncology drug mocravimod in phase 2b/3 for acute myeloid leukaemia (AML) in partnership with Kyowa Kirin.

Camargo
OCTOBER 14, 2020
The second guidance, “ ANDA Submissions – Amendments and Requests for Final Approval to Tentatively Approved ANDAs ,” deals with the not uncommon situation wherein an ANDA application has received tentative approval because of a Paragraph III patent certification in the original application.

FDA Law Blog
JUNE 13, 2024
Though some drug companies have been reluctant to delist certain patents from the Orange Book, the District Court of New Jersey just ordered Teva to delist 5 of its patents that it deemed improperly listed. The Court addressed Teva’s valid argument that the Inhaler Patents are drug product patents and thus listable.

XTalks
AUGUST 30, 2022
According to an analysis of the 2013 Medical Expenditure Panel Survey, one in six US adults reported taking a psychiatric drug at least once in 2013. CNS drugs treat a range of neurologic and psychiatric disorders, such as psychosis, depression, Parkinson’s disease, multiple sclerosis and epilepsy.

XTalks
NOVEMBER 3, 2021
The team also learned about the FDA approval of Genentech’s new ocular implant drug delivery system, called Susvimo, for the treatment of neovascular, or wet, age-related macular degeneration (nAMD). Read the full articles here: FDA Authorizes Pfizer/BioNTech COVID-19 Vaccine for Kids 5 to 11.

pharmaphorum
NOVEMBER 7, 2022
SAE Media Group reports: Mitchell de Long, Vice President, Chemistry from Aerie Pharmaceuticals and Naj Sharif, Vice President, Ophthalmology Innovation Center from Santen Inc USA cordially invite industry experts to speak at the 5th Annual Ophthalmic Drugs Conference. Ophthalmic Drugs. This event is less than four weeks away.

XTalks
MAY 18, 2023
” XTALKS WEBINAR: Drug Delivery — Addressing Global Regulatory Challenges in Co-Packaged, On-Body Systems Live and On-Demand: Thursday, June 15, 2023, at 10am EDT (4pm CEST/EU-Central) Register for this free webinar to learn about the differences and similarities between the US and EU regulatory frameworks for co-packaged combination products.
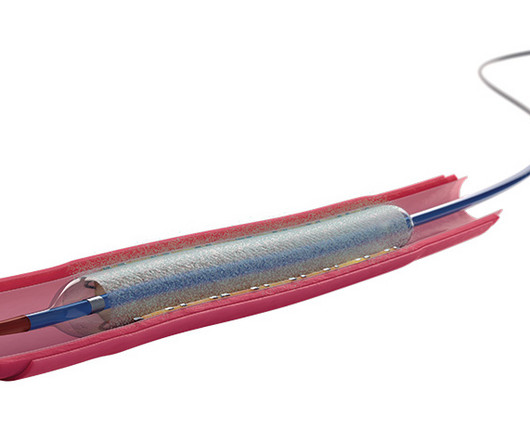
XTalks
JUNE 11, 2024
The US Food and Drug Administration (FDA) recently approved Boston Scientific’s Agent drug-coated balloon (DCB) for treating coronary in-stent restenosis (ISR) in patients with coronary artery disease (CAD). Repeat PCI: For patients experiencing restenosis, a repeat PCI procedure can be performed.

FDA Law Blog
SEPTEMBER 19, 2023
The six-page statement explains that “Brand drug manufacturers may be harming generic competition through the improper listing of patents in the. Of course, the statute says that only drug formulation, composition, or method of use patents are listable, but FDA has not defined the scope of the “drug” that must be covered by the patent.
pharmaphorum
MARCH 16, 2022
Viatris (formerly Mylan) has become the first drugmaker to win full FDA approval for a generic version of AstraZeneca’s top-selling respiratory drug Symbicort, although launch plans remain uncertain for now.

Roots Analysis
FEBRUARY 22, 2022
Further, in order to improve the quality of life in these patients, companies have developed novel devices capable of delivering a variety of formulations of different drugs / therapies, in an efficient and relatively simple manner. Autoinjector As Emerging Drug Delivery Device.
Delveinsight
NOVEMBER 26, 2020
Retinal vein occlusion drug market. The treatment options such as Intravitreal injection of anti-vascular endothelial growth factor (VEGF) drugs, and Intravitreal injection of corticosteroid drugs. Intraocular injections of steroids are another potential treatment for eyes that don’t respond to anti-VEGF drugs.
The Pharma Data
OCTOBER 26, 2020
Food and Drug Administration (FDA) has approved EYSUVIS for the short-term treatment of dry eye disease. . EYSUVIS is the first FDA-approved corticosteroid specifically for dry eye disease treatment. The FDA approved EYSUVIS based on the positive results from one Phase II and three Phase III trials.

FDA Law Blog
MARCH 12, 2024
Koblitz — After years of silence from FDA on whether certain patents could be listed in the Orange Book, some manufacturers of drug and device combination products have had a rude awakening lately.
Delveinsight
NOVEMBER 29, 2020
Looking at the potential of the pipeline therapies they hold once approved, pharmaceutical companies are charting out a seemingly exciting course in the market. Therapies such as dexamethasone drug delivery system and vascular endothelial growth factor inhibitors have already pierced deeper into the market.

XTalks
MAY 30, 2024
Potential New CIDP Treatment: argenx’s Efgartigimod Alfa The US Food and Drug Administration (FDA) has accepted argenx’s supplemental Biologics License Application (sBLA) for Vyvgart Hytrulo (efgartigimod alfa and hyaluronidase-qvfc) for priority review to treat CIDP.

The Pharma Data
JANUARY 17, 2021
a biopharmaceutical company engaged in the discovery and development of RNAi therapeutics against cancer and fibrotic diseases, today announced dose administration for the first patient in a Phase 2a clinical study of the company’s lead drug candidate, STP705, for the treatment of cutaneous basal cell carcinoma. GAITHERSBURG, Md.

The Pharma Data
JANUARY 6, 2021
The approach used to turn botulinum toxin into a kind of Trojan horse that delivers a cargo into neurons has enormous potential for future drug development,” noted Thomas C. “This is a landmark study in converting the power of lethal botulinum neurotoxins into therapies. Südhof, M.D.,
XTalks
NOVEMBER 5, 2024
Its lead candidate, PL-14, targets nasal allergies and is scheduled for US Food and Drug Administration (FDA) submission via the 510(k) pathway, with preclinical trials expected to start in the second quarter of 2025 and pivotal trials by the end of 2025.

The Pharma Data
JANUARY 17, 2021
Food and Drug Administration (FDA) approved Janssen Pharmaceuticals ’ (a Johnson and Johnson company) Darzalex Faspro for adults with newly diagnosed light chain amyloidosis. It was approved in combination with bortezomib, cyclophosphamide and dexamethasone (D-VCd). Michael Vi/Shutterstock.

XTalks
JANUARY 18, 2023
Ayesha also talked about the FDA approval of AstraZeneca’s asthma inhaler Airsupra, the first-in-class drug that contains both a beta agonist and corticosteroid. The inhaler is approved as a rescue medication for adults with asthma to help treat bronchoconstriction and asthma attacks.

pharmaphorum
JUNE 22, 2021
GlaxoSmithKline (GSK) has reported encouraging data for its COVID-19 antibody sotrovimab, and an alliance with Halozyme to develop a new generation of long-acting HIV drugs, as it prepares to give a much-anticipated update to shareholders tomorrow. So far, all the other antibodies are also delivered by intravenous infusion.

The Pharma Data
AUGUST 31, 2021
Additionally, Janssen will present an update on the Phase 3 SunRISe-2 trial evaluating an investigational intravesical drug delivery system, TAR-200, in combination with the programmed cell death receptor-1 (PD-1) inhibitor cetrelimab in muscle-invasive urothelial carcinoma (Abstract # MP13-17). [1]. DRUG INTERACTIONS.

The Pharma Data
JANUARY 15, 2021
Food and Drug Administration (FDA) approval of Darzalex Faspro ® (daratumumab and hyaluronidase-fihj), a subcutaneous formulation of daratumumab, in combination with bortezomib, cyclophosphamide and dexamethasone (D-VCd) for the treatment of adult patients with newly diagnosed light chain (AL) amyloidosis.[1] Haematologica.
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?


Let's personalize your content