Nxera and Cancer Research UK to present promising cancer drug trial at ESMO
Pharma Times
SEPTEMBER 13, 2024
Innovative immunotherapy drug HTL0039732 shows potential in trials
This site uses cookies to improve your experience. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country, we will assume you are from the United States. Select your Cookie Settings or view our Privacy Policy and Terms of Use.
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.
Used for the proper function of the website
Used for monitoring website traffic and interactions
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.

Pharma Times
SEPTEMBER 13, 2024
Innovative immunotherapy drug HTL0039732 shows potential in trials

pharmaphorum
FEBRUARY 21, 2024
Clinical research involves trial and error as part of the drug development process. Learn more about how to optimise drug trials and the important role of participants in clinical research.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Pharma Times
AUGUST 22, 2024
200,000 people in the UK have what is considered a ‘hidden’ lung condition

pharmaphorum
MAY 19, 2022
Phase 2 trials of Beckley Psytech’s psychedelic drug candidates will make use of digital technologies for patient monitoring, thanks to a new partnership with Empatica. The post Beckley adds digital element to its psychedelic drug trials appeared first on.

pharmaphorum
JANUARY 25, 2021
GlaxoSmithKline has suffered another research setback after it axed a phase 2 trial of an anti-LAG3 drug in patients with ulcerative colitis, following a major disappointment with a key lung cancer drug last week. The post GSK suffers another R&D setback, axing ulcerative colitis drug trial appeared first on.

STAT News
MARCH 29, 2023
And hopeful patients, many of them young women of color, are waiting — just three lupus drugs have been approved by the Food and Drug Administration in six decades. On Wednesday, the nonprofit Lupus Research Alliance announced it was entering into a public-private partnership with the FDA.

Pharma Times
AUGUST 15, 2024
Children accounted for the majority of mpox cases and deaths in the DRC since June 2023

pharmaphorum
MAY 7, 2021
Small Pharma is following the trend for development of psychedelic medicines, a field where research has been suppressed for years because of legal restrictions on use of these compounds in many countries. The goal of the trial is for proof of concept of the drug as a wrap-around therapy for Major Depressive Disorder.

Scienmag
JANUARY 19, 2022
New research suggests manufacturers of newly developed antidepressant drugs have become more forthcoming about clinical trials that don’t pan out.

Medical Xpress
DECEMBER 29, 2022
A new drug designed to ward off Alzheimer's is being tested at more than 100 sites around the world, including the University of Pennsylvania.

STAT News
FEBRUARY 17, 2023
Pfizer and partner Valneva are scrapping data from roughly half of the participants in a Phase 3 trial of a Lyme disease vaccine candidate after uncovering quality issues with a third-party clinical trial operator.

BioPharma Reporter
JULY 11, 2024
Amid a period of clinical holds and legal tangles, the US antibody specialist CytoDyn has reached a settlement to resolve legal disputes with its former contract research organization (CRO) Amarex Clinical Research.

Pharmaceutical Technology
JUNE 15, 2023
The need for new medical treatments and drugs has never been greater. But before pharmaceutical companies can go to market with a breakthrough drug, they need to ensure safety and efficacy through clinical trials. Even reaching the clinical trial phase offers no guarantees, as only 12% of such drugs receive U.S.
World of DTC Marketing
APRIL 20, 2022
This was especially true with new drugs that have a significant competitive market. Trust in pharma did not decline, but they don’t want to be told about the data via charts; they want the ability to read the data for themselves and talk to other HCPs about the data and drug.

pharmaphorum
AUGUST 4, 2020
New research looks at the factors that speed up and slow down HTA appraisals for rare disease medicines across Europe. Rare diseases drugs have always faced challenges when it comes to HTA approvals, even as governments bring in more regulatory policies that make their path through assessment easier. Source: CRA Analysis.
pharmaphorum
JANUARY 19, 2021
Sites need to be close enough for travel, and the number of site visits and trial appointments must also be manageable for people living with the complications of having little-known and rarely-researched orphan diseases. In fact, how do rare disease patients even feel about clinical trials?

Scienmag
JULY 18, 2021
Researchers are using computer models to simulate COVID-19 infections on a cellular level – the basic structural level of the human body. The models allow for virtual trials of drugs and vaccines, opening the possibility of pre-assessment for drug and vaccine efficacy against the virus.

Outsourcing Pharma
NOVEMBER 1, 2021
A former employee of Tellus Clinical Research in Miami has admitted to charges related to a conspiracy to falsify data on a list of clinical drug trials.

pharmaphorum
AUGUST 6, 2020
Lundbeck has axed a phase 2 trial of a schizophrenia drug, saying that the study was unlikely to meet its efficacy target. The company said it made the decision after a futility analysis indicated that the trial was not likely to succeed, when measured against a standard scale.
pharmaphorum
MAY 14, 2021
China’s Kintor Pharmaceutical has begun late-stage US clinical development of its potential COVID drug proxalutamide – but there is scepticism over the company’s claims about its research. Reuters quoted Kintor’s chief financial officer Lucy Lu, who said that Kayali was one of its trial investigators.

Scienmag
JULY 8, 2021
Researchers, including academics from the University of York, analysed systematic reviews of 1,200 Randomised Controlled Trials (RCTs) to assess whether reporting had improved over time.
STAT News
DECEMBER 2, 2022
And a fragmented clinical trials industry has made it a prime target for private equity, which often consolidates markets by merging companies. To date, 11 of the 25 private equity firms identified by industry tracker PitchBook as the top investors in health care have bought stakes in clinical research companies.

XTalks
JULY 29, 2021
Just as clinical researchers in other therapeutic areas have renewed their commitment to improving participant diversity in clinical trials, so too have those working in Alzheimer’s research. Fortunately, there are a number of researchers working on this issue. Similarly, older Latino and Hispanic individuals are 1.5
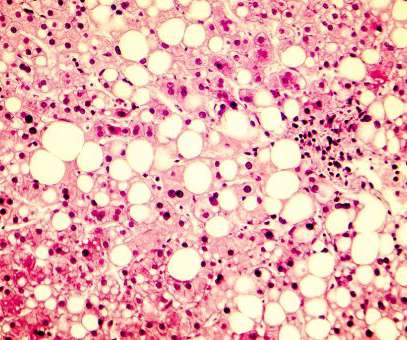
pharmaphorum
AUGUST 25, 2022
Cirrhosis is not just an “old drunk guys” disease, a stigma which has long hampered interest and investment in research. But herein lies the dichotomy that medicine has created: By failing to regularly screen to find the early-stage disease, the number of patients who could participate in drug trials is limited.

BioTech 365
JULY 19, 2021
Biotechnology, Pharma and Biopharma News – Research – Science – Lifescience ://Biotech-Biopharma-Pharma: Troubled Cytokinetics’ stock soars as gets much needed phase 2 heart drug trial win, plans late-stage effort by year’s end.Troubled Cytokinetics’ stock soars as gets much needed … Continue reading →

pharmaphorum
FEBRUARY 19, 2021
million, which uses artificial intelligence (AI) technology to speed up drug development. With the acquisition MindMed gains access to HealthMode’s intellectual property, platforms for clinical drug trials, and its entire 24-strong digital medicine team.

The Pharma Data
JANUARY 20, 2021
Drug Industry Daily (DID) the premier online resource for savvy pharmaceutical professionals whose jobs depend on accurate knowledge about the daily activities of Congress, the FDA, other key regulators … and what their competitors are up to. Become a subscriber to Drug Industry Daily today. No questions asked.
Medical Xpress
DECEMBER 21, 2022
An international research group including scientists from Italy, the United States, Ireland, and Israel have published a three-year analysis of the Mesothelioma (Me) drug trial, Check-Mate 743 (CM-743).

The Pharma Data
OCTOBER 26, 2020
based aTyr Pharma has finalized enrollment for a phase 2 trial to evaluate an investigational anti-inflammatory drug in COVID-19 patients with severe respiratory complications. San Diego, Calif.-based ATYR1923 is meant to reduce inflammation and restore the lung’s immune balance.
Advarra
JUNE 27, 2023
Clinical trials are studies involving human volunteers to research ways to prevent, detect, and treat various medical conditions. The goal is to identify safer, more effective methods of treating illnesses using new drugs or new combinations of existing drugs.

VirTrial
MAY 26, 2020
The solution enables pharmaceutical sponsors and CROs to evaluate, qualify and routinely monitor research sites for studies without physical travel. COVID-19 spurred a significant increase in Virtual Pre-Site inquiries to accommodate site monitoring for existing studies and to expedite study startups for COVID and other drug trials.

BioSpace
APRIL 1, 2021
One of the common research approaches to treating brain diseases such as Parkinson’s disease (PD) and Alzheimer’s disease (AD) is using antibodies designed to clear the accumulated misfolded proteins implicated in the diseases. To date, these drug trials have not been particularly successful at i.

Worldwide Clinical Trials
NOVEMBER 3, 2022
BioPharma Dive interviewed our Derek Ansel on how to conduct psychedelic clinical research. In April 2022, the Food and Drug Administration (FDA) issued draft guidance on developing a “Race and Ethnicity Diversity Plan” for clinical trials. But in rare disease trials, that can be challenging given the barriers inherent in U.S.

Pharmaceutical Technology
SEPTEMBER 20, 2022
Unlike commercial pharmaceutical packaging, the primary consideration in clinical trial packaging is protecting the product quality and reliability for research. Furthermore, the list includes clinical supply packaging, as well as labelling, storage, and logistics service providers for drug trial projects.

pharmaphorum
OCTOBER 27, 2020
Analysing real-world health data could help overcome the bias towards men in traditional medical research, says Sensyne Health’s Dr Lucy Mackillop. Medical research has often focused on men, meaning that the insights gained have not always been reflective of how women would react to a treatment or disease.

The Pharma Data
NOVEMBER 30, 2020
Sponsors of drug trials should determine what effect the use of acid-reducing agents (ARA), such as antacids, proton pump inhibitors and histamine blockers, could have on the solubility of an orally administered drug, according to a new FDA draft guidance released Monday.

The Pharma Data
DECEMBER 8, 2020
The company also said the hold does not interfere with enrollment of an upcoming phase 1/2 trial evaluating similar CAR-T immunotherapy drug BPX-603 in breast cancer patients with solid tumors. The Houston, Tex., Source link.

pharmaphorum
OCTOBER 1, 2020
Researchers at the University of Oxford in the UK have started a trial of TNF inhibitor adalimumab for COVID-19 patients in care homes and other community settings.

Velocity Clinical Research
OCTOBER 21, 2024
At the beginning of September, Velocity Clinical Research topped Business North Carolina ’s 2024 Fast 40 list. For a clinical trials organization to achieve this level of scale is notable. This is vital if we’re to ensure that drug trials are inclusive and deliver results that are representative of the entire population.

pharmaphorum
SEPTEMBER 27, 2021
As people have become more conscious and vocal about diversity and inclusion, Page & Page’s Lisa Lishman says pharma should reassess how it conducts clinical trials. The pharmaceutical industry is one of the most important industries in the world, providing lifesaving drugs and treatment to people who need it the most.
XTalks
MAY 16, 2023
Here’s an overview of clinical trials for pulmonary fibrosis: Drug trials: These trials may involve investigational drugs targeting specific pathways involved in fibrosis or assessing the efficacy of approved drugs in combination therapy.

pharmaphorum
DECEMBER 1, 2022
A recent survey of clinical trial professionals found that more than two-thirds of respondents (61%) believe giving patients choice will have a positive impact on clinical research, and well over half (58%) said that their organisations plan to give patients the option to choose how they participate in clinical trials moving forward.

XTalks
SEPTEMBER 11, 2020
Clinical Trials Don’t Reflect Demographics. Disease doesn’t discriminate, and researchers understand different populations react differently to drugs. Why, then, are clinical trials so stubbornly homogeneous? Clinical trials, however, don’t reflect that diversity. Why aren’t clinical trials more inclusive?

Delveinsight
AUGUST 13, 2021
Clinical trials are an essential part of the drug development process. Worldwide, more than 2 million clinical trials are registered and the number is steadily increasing over the past several years. The conduct of trials was not possible due to the risk of catching coronavirus. Why the Need?
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?


Let's personalize your content