Springworks shares fall despite drug trial's success
Bio Pharma Dive
MAY 24, 2022
The biotech plans to seek FDA approval for its soft-tissue tumor treatment after positive study results. But investors still sent the stock down by nearly 10%.

Bio Pharma Dive
MAY 24, 2022
The biotech plans to seek FDA approval for its soft-tissue tumor treatment after positive study results. But investors still sent the stock down by nearly 10%.

Delveinsight
AUGUST 13, 2021
Clinical trials are an essential part of the drug development process. Worldwide, more than 2 million clinical trials are registered and the number is steadily increasing over the past several years. The conduct of trials was not possible due to the risk of catching coronavirus. What are Virtual Clinical Trials?
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

XTalks
AUGUST 3, 2022
In this episode, Ayesha discussed the FDA approval of Azurity Pharmaceutical’s Zonisade (zonisamide oral suspension) as an adjunct therapy for the treatment of seizures in adults and pediatric patients 16 years of age and older with epilepsy. The drug is the first FDA approved oral suspension form of zonisamide.
XTalks
AUGUST 4, 2022
Cardiovascular clinical trials need to have a diversity plan because there can be a difference in disease burden when comparing people of different race and ethnicity. The US Food and Drug Administration (FDA) is emphasizing the need for diversity in clinical trials. The Diversity of the US Population.
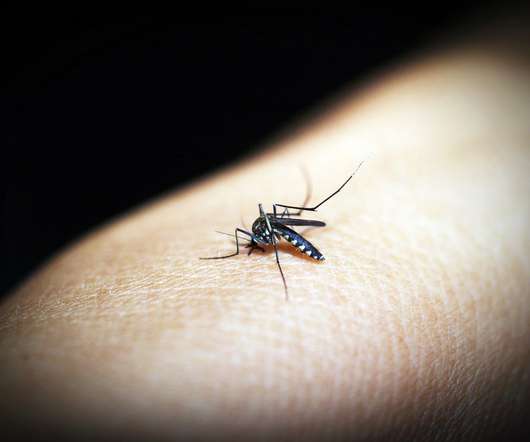
XTalks
APRIL 26, 2021
After years of disappointing malaria vaccine trials, a malaria shot developed by researchers at the Jenner Institute at the University of Oxford has demonstrated an unprecedentedly high efficacy of 77 percent, and may be the magic bullet the world has been waiting for against the deadly disease. Malaria Vaccine Phase II Trial.

Delveinsight
AUGUST 12, 2021
Takeda leads Finch-partnered Microbiome IBD drug. When Takeda collaborated with Finch Therapeutics on inflammatory bowel disease (IBD) , it intended to pick up programs after they had finished phase 2 trials. Finch will proffer ongoing technical support through the phase 1 trial of the treatment.

Advarra
AUGUST 16, 2022
Orphan drugs have historically faced a number of barriers, such as limited research and development (R&D) investment due to an expected lack of profitability as well as challenges in clinical trial design and recruitment. Before 1983, only 38 orphan drugs had received U.S. Food and Drug Administration (FDA) approval.
Let's personalize your content