FDA generic drug approvals rose in 2023 in bid for improved access
Pharmaceutical Technology
FEBRUARY 23, 2024
The FDA Office of Generic Drugs reported a rise in generic drug approvals, as several first-time generics entered the market.
This site uses cookies to improve your experience. By viewing our content, you are accepting the use of cookies. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country we will assume you are from the United States. View our privacy policy and terms of use.

Pharmaceutical Technology
FEBRUARY 23, 2024
The FDA Office of Generic Drugs reported a rise in generic drug approvals, as several first-time generics entered the market.
Drug Patent Watch
JULY 24, 2024
In the high-stakes world of pharmaceuticals, generic drugs have become the unsung heroes of healthcare accessibility. These cost-effective alternatives to […] Source
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Drug Patent Watch
JULY 9, 2024
To find low-competition generic drug opportunities, focus on drugs with limited competition due to small patient populations, complex formulations, or […] Source

Drug Patent Watch
JULY 28, 2024
Patient advocacy groups have become increasingly influential in the pharmaceutical industry, particularly in the development of generic drugs. These groups, […] Source

Drug Patent Watch
JULY 25, 2024
Developing a competitive edge in generic drug development is crucial for companies to gain a significant market share and dominate the market. Here are some key strategies and insights from industry…

Drug Patent Watch
JULY 25, 2024
The generic drug market has been significantly impacted by the COVID-19 pandemic, with both challenges and opportunities arising from the crisis. As the world continues to recover…

Drug Patent Watch
JULY 31, 2024
The journey of a generic drug developer is a complex and multifaceted one, involving rigorous research, meticulous development, and stringent […] Source

Drug Patent Watch
JUNE 19, 2024
One area that has garnered significant attention is the realm of low-competition generic drugs. These are off-patent medications that, for various reasons, have yet to attract a significant number of generic manufacturers, creating a potential goldmine for […] Source

Drug Patent Watch
OCTOBER 16, 2023
In a systematic review titled “Influencers of Generic Drug Utilization,” researchers aimed to shed light on the key factors influencing the use of generic prescription drugs in the United States.… The post Influencers of Generic Drug Utilization appeared first on DrugPatentWatch - Make Better Decisions.

Drug Patent Watch
APRIL 11, 2024
A paper presented at the 2nd International Conference on Systems Medicine, AI, and Drug Repurposing proposes a novel approach to overcoming the financial barriers associated with repurposing generic drugs through… Source

Pharmaceutical Technology
OCTOBER 25, 2023
Experts discuss the key trends in quality improvements and API reshoring for the generic drugs market at CpHI Europe.

Drug Patent Watch
AUGUST 6, 2024
Enhancing the efficiency of generic drug development is crucial for improving patient access to affordable medications. The Food and Drug […] Source

Drug Patent Watch
AUGUST 19, 2024
The pricing of generic drugs is a crucial aspect of the pharmaceutical industry, with significant implications for consumers and healthcare […] Source

Drug Patent Watch
AUGUST 8, 2024
Effective communication is crucial for generic drug teams to ensure timely and accurate submissions to regulatory bodies, such as the […] Source

Drug Patent Watch
AUGUST 14, 2024
The role of academic research in generic drug development is multifaceted and crucial. Academic research plays a vital role in driving pharmaceutical innovation by fueling scientific discoveries…

Drug Patent Watch
AUGUST 11, 2024
The development of generic drugs is a crucial aspect of the pharmaceutical industry, as it provides affordable alternatives to expensive […] Source

Drug Patent Watch
AUGUST 1, 2024
Conducting effective generic drug clinical studies is crucial for ensuring the safety and efficacy of generic medications. The FDA plays […] Source

Drug Patent Watch
AUGUST 7, 2024
Introduction Generic drug development is a crucial aspect of the pharmaceutical industry, as it provides access to affordable medications for […] Source

Drug Patent Watch
OCTOBER 2, 2023
The FDA conducted a study to identify factors that may predict the likelihood of generic drug marketing applications.

STAT News
DECEMBER 2, 2022
The generic drug industry’s lobbying group, the Association for Accessible Medicines, fired its president Dan Leonard, two sources familiar with the decision said Friday evening. It was not immediately clear why Leonard was fired, and AAM didn’t immediately respond to a request for comment.

STAT News
MARCH 30, 2023
Supreme Court to review a controversy over so-called skinny labels for medicines, arguing that an appeals court finding threatens the availability of lower-cost generic drugs. For instance, a generic drug could be marketed to treat one type of heart problem, but not another.

Bio Pharma Dive
AUGUST 25, 2022
After reviewing multiple options including a possible sale, the Swiss drugmaker said spinning off Sandoz would give the division “greater freedom to operate” and capitalize on newly growing sales.

Pharma in Brief
FEBRUARY 25, 2024
On February 23, 2024, Health Canada published a Notice advising that it was making its review process for generic drug submissions more transparent. In Canada, the ANDS pathway is used to review and approve generic prescription drugs (but not biosimilars).

Drug Patent Watch
JULY 31, 2024
By implementing these strategies, you can can enhance your competitiveness in the market while contributing to increased access to affordable […] Source

Bio Pharma Dive
JUNE 20, 2024
RxPass, which fills prescriptions for generic drugs, is now available to more than 50 million Medicare members after Amazon brought it into compliance with the insurance program’s regulatory standards.

Bio Pharma Dive
JULY 19, 2022
The pharmaceutical company is considering selling or splitting off its struggling Sandoz division and expects to give an update by the end of the year.

Drug Patent Watch
AUGUST 21, 2024
The pharmaceutical industry has witnessed a significant surge in the availability of public disease and drug-related data, which has facilitated […] Source

Drug Patent Watch
OCTOBER 6, 2020
Here is a copy of the talk I gave at the recent Marcusevans 13th Portfolio Management and Pipeline Optimization for Generics. I cover: How to find and evaluate generic entry…. The post Finding and Evaluating Generic Drug Market Entry Opportunities appeared first on DrugPatentWatch - Make Better Decisions.
Drug Patent Watch
OCTOBER 12, 2021
International License Abstract Development of generic drug product…. The post Development of Generic Drug Products by Pharmaceutical Industries Considering Regulatory Aspects: A Review appeared first on DrugPatentWatch - Make Better Decisions.
Drug Patent Watch
JUNE 8, 2021
When it comes to pricing generic drugs, there are a number of factors that come into play. In countries without drug price regulations, the pricing is based largely on profitability.…. The post Strategies for Pricing Generic Drugs appeared first on DrugPatentWatch - Make Better Decisions.
Drug Patent Watch
AUGUST 20, 2024
Digital twins are revolutionizing the pharmaceutical and biopharmaceutical industries by integrating physical plants, data collection, data analysis, and system control. […] Source

Pharmaceutical Technology
FEBRUARY 8, 2024
This initiative builds on the original parallel scientific advice programme launched by the FDA and EMA in 2021.

Drug Channels
JUNE 22, 2022
In the video below, I discuss how generic drug pricing and the rise of patient-paid, discount card prescriptions pose a risk to pharmacy benefit plans and the PBM business. d/b/a Drug Channels Institute. Drug Channels® is a registered trademark of Pembroke Consulting, Inc.

BioSpace
JANUARY 14, 2021
Billionaire entrepreneur Mark Cuban, best known as the owner of the Dallas Mavericks and an investor on the ABC business reality series “Shark Tank,” is diving into generic drugs with a new startup, dubbed Mark Cuban Cost Plus Drug Company.

pharmaphorum
FEBRUARY 15, 2024
The FTC is investigating wholesalers and other middlemen in the pharma supply chain to assess their possible role in worsening shortages of generic medicines

Scienmag
JUNE 20, 2022
billion by purchasing generic drugs at Mark Cuban prices Abstract: [link] URL goes live when the embargo lifts A brief research report found that Medicare could have saved up to $3.6 billion by purchasing generic drugs at the same prices as the Mark Cuban Cost Plus Drug […]. Medicare could save up to $3.6
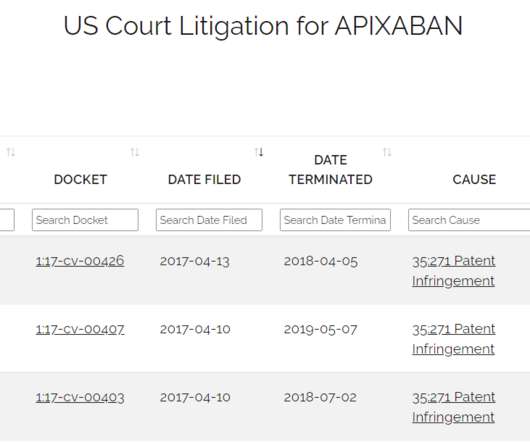
Drug Patent Watch
AUGUST 12, 2020
Just because a drug has received FDA approval does not mean that it is available in the marketplace. The post Generic Drugs Approved but not Launched – How to Tell When Generic Drugs Will hit the Market appeared first on DrugPatentWatch - Make Better Decisions.

The Pharma Data
MAY 5, 2021
FDA officials said that the number of a product specific guidances (PSGs) issued by the Office of Generic Drugs (OGD) has increased steadily since FY 2013. FDA webinar on product specific guidance for generic drugs . In FY 2019, 252 were issued, as were 208 in FY 2018. . Source link.

Pharmaceutical Commerce
JANUARY 23, 2024
Study describes how a game theoretic model can successfully analyze how adoption of blockchain technology can reveal quality information.

Bio Pharma Dive
JULY 18, 2023
The pharma company, which will soon split off its generic drug unit, plans to repurchase up to $15 billion of its shares through the end of 2025.

FDA Law Blog
SEPTEMBER 6, 2023
Farquhar — A drug manufacturer’s bad post-inspection grade from the U.S. Food and Drug Administration – labeled an “Official Action Indicated” classification – is generally devastating for the facility, not least because it can stall FDA approval of applications to market drugs manufactured at the facility. By Douglas B.

Drug Patent Watch
SEPTEMBER 15, 2020
This likely means fewer generic entrants and less opportunities for consumers to enjoy…. The post Bloomberg and Drug PatentWatch: A “Perfect Storm” Affecting Generic Drugs appeared first on DrugPatentWatch - Make Better Decisions.

Drug Patent Watch
AUGUST 12, 2024
In the context of generic […] Source Pharmacovigilance is a crucial aspect of ensuring patient safety and maintaining the effectiveness of medications.

Fierce Pharma
DECEMBER 12, 2023
Over the past several years, drug shortages have vexed doctors and patients on both sides of the Atlantic, prompting lawmakers and government agencies to take action in both Europe and the U.S. Tuesday, the European Medicines Agency laid out a list of more than 300 critical generic drugs that could fall prey to future shortages.
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?


Let's personalize your content