FDA generic drug approvals rose in 2023 in bid for improved access
Pharmaceutical Technology
FEBRUARY 23, 2024
The FDA Office of Generic Drugs reported a rise in generic drug approvals, as several first-time generics entered the market.
This site uses cookies to improve your experience. By viewing our content, you are accepting the use of cookies. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country we will assume you are from the United States. View our privacy policy and terms of use.

Pharmaceutical Technology
FEBRUARY 23, 2024
The FDA Office of Generic Drugs reported a rise in generic drug approvals, as several first-time generics entered the market.

Drug Patent Watch
SEPTEMBER 26, 2024
The generic drug market is a complex and dynamic environment where multiple factors influence the availability, quality, and pricing of generic drugs. This article will discuss the key strategies and… Source
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Drug Patent Watch
SEPTEMBER 30, 2024
Patent expirations have a significant impact on the pharmaceutical industry, particularly on the generic drug market. When a drug’s patent expires, other manufacturers can produce and market generic versions of the drug, leading to increased competition and lower prices.

Drug Patent Watch
OCTOBER 10, 2024
China has emerged as a significant player in the global generic drug active pharmaceutical ingredient (API) market. This article will delve into the role of China in the global generic drug API market, exploring its market share… Source

Drug Patent Watch
NOVEMBER 18, 2024
The pharmaceutical industry is undergoing a significant shift, with emerging markets offering the next growth opportunity. This growth is driven by several factors, including the increasing prevalence of chronic diseases, cost-effectiveness, and patent expirations of branded drugs.
Drug Patent Watch
SEPTEMBER 23, 2024
The generic drug market has experienced significant growth over the past few decades, driven by the expiration of patents on branded drugs and the increasing demand for affordable healthcare solutions. To succeed in this market, generic drug manufacturers must adopt innovative… Source

Pharmaceutical Technology
OCTOBER 25, 2023
Experts discuss the key trends in quality improvements and API reshoring for the generic drugs market at CpHI Europe.

Drug Patent Watch
JULY 25, 2024
The generic drug market has been significantly impacted by the COVID-19 pandemic, with both challenges and opportunities arising from the crisis. As the world continues to recover…

Drug Patent Watch
SEPTEMBER 10, 2024
The generic drug market has experienced significant growth over the past few decades, driven by the passage of the Hatch-Waxman Act in 1984 and subsequent legislation. Today, the market is more competitive than ever, with generic drugs accounting for over 90% of all prescriptions in the United States.

Drug Patent Watch
SEPTEMBER 19, 2024
The generic drug market in the United States is characterized by significant price volatility and shortages, driven by the structure of the market and the incentives for manufacturers. To address these issues, several market-based proposals have been put forth to optimize generic drug cost and availability.

Drug Patent Watch
JULY 25, 2024
Developing a competitive edge in generic drug development is crucial for companies to gain a significant market share and dominate the market. Here are some key strategies and insights from industry…

Drug Patent Watch
OCTOBER 2, 2023
The FDA conducted a study to identify factors that may predict the likelihood of generic drug marketing applications.

Drug Patent Watch
JUNE 19, 2024
In the ever-evolving pharmaceutical landscape, savvy businesses are constantly on the lookout for untapped markets and lucrative opportunities. One area that has garnered significant attention is the realm of low-competition generic drugs.

STAT News
MARCH 30, 2023
Supreme Court to review a controversy over so-called skinny labels for medicines, arguing that an appeals court finding threatens the availability of lower-cost generic drugs. For instance, a generic drug could be marketed to treat one type of heart problem, but not another.

Drug Patent Watch
OCTOBER 6, 2020
Here is a copy of the talk I gave at the recent Marcusevans 13th Portfolio Management and Pipeline Optimization for Generics. I cover: How to find and evaluate generic entry…. The post Finding and Evaluating Generic Drug Market Entry Opportunities appeared first on DrugPatentWatch - Make Better Decisions.
BioSpace
JANUARY 14, 2021
Billionaire entrepreneur Mark Cuban, best known as the owner of the Dallas Mavericks and an investor on the ABC business reality series “Shark Tank,” is diving into generic drugs with a new startup, dubbed Mark Cuban Cost Plus Drug Company.

Drug Patent Watch
JULY 31, 2024
By implementing these strategies, you can can enhance your competitiveness in the market while contributing to increased access to affordable […] Source
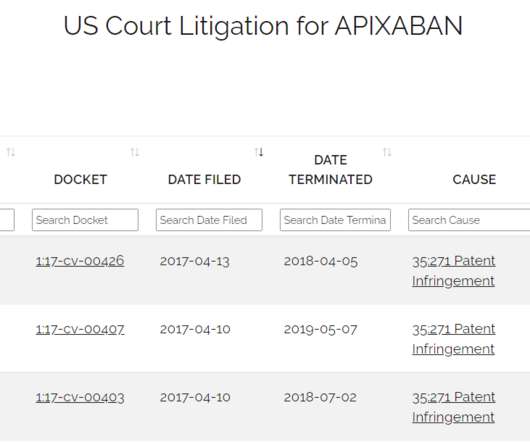
Drug Patent Watch
AUGUST 12, 2020
Just because a drug has received FDA approval does not mean that it is available in the marketplace. The post Generic Drugs Approved but not Launched – How to Tell When Generic Drugs Will hit the Market appeared first on DrugPatentWatch - Make Better Decisions.

BioTech 365
JUNE 25, 2021
GCC Generic Drug Market Trends, Share, Size, Opportunity and Forecasts 2021-2026 – ResearchAndMarkets.com GCC Generic Drug Market Trends, Share, Size, Opportunity and Forecasts 2021-2026 – ResearchAndMarkets.com DUBLIN–(BUSINESS WIRE)–The “GCC Generic Drug Market: Industry Trends, Share, Size, Growth, Opportunity and Forecast (..)

BioTech 365
MAY 28, 2021
Global Generic Drugs Market Report 2021: The New Generics Era – The Patent Cliff, Types of Generic Drugs, Simple Generics, Super Generics, Biosimilars, ANDA Approvals – ResearchAndMarkets.com Global Generic Drugs Market Report 2021: The New Generics Era – The Patent … Continue reading →

BioTech 365
OCTOBER 4, 2021
Canada Generic Drug Market Industry Trends, Share, Size, Growth, Opportunity and Forecasts 2021-2026 – ResearchAndMarkets.com Canada Generic Drug Market Industry Trends, Share, Size, Growth, Opportunity and Forecasts 2021-2026 – ResearchAndMarkets.com DUBLIN–(BUSINESS WIRE)–The “Canada Generic Drug Market: Industry Trends, (..)

BioTech 365
NOVEMBER 18, 2021
Brazil Generic Drug Market (2021 to 2026) – Industry Trends, Share, Size, Growth, Forecast and Opportunities – ResearchAndMarkets.com Brazil Generic Drug Market (2021 to 2026) – Industry Trends, Share, Size, Growth, Forecast and Opportunities – ResearchAndMarkets.com DUBLIN–(BUSINESS WIRE)–The “Brazil Generic (..)

NY Times
SEPTEMBER 18, 2021
Competition for market share at rock-bottom prices has led to shortages, price-spikes, allegations of price-fixing, and substandard and even dangerous practices.

BioTech 365
APRIL 15, 2021
Global Generic Drugs (Small Molecule Generics vs Biosimilars) Market Forecast & Opportunities, 2021-2026 by Application, Drug Delivery, Form, Source, Distribution Channel – ResearchAndMarkets.com Global Generic Drugs (Small Molecule Generics vs Biosimilars) Market Forecast & Opportunities, 2021-2026 by Application, Drug Delivery, … (..)

BioTech 365
APRIL 26, 2021
United States Generic Drugs Market Report 2020-2026: Focus on CNS, Cardiovascular, Dermatology, Genitourinary/Hormonal, Respiratory, Anti-infective & Oncology – ResearchAndMarkets.com United States Generic Drugs Market Report 2020-2026: Focus on CNS, Cardiovascular, Dermatology, Genitourinary/Hormonal, Respiratory, Anti-infective & (..)

FDA Law Blog
SEPTEMBER 6, 2023
Farquhar — A drug manufacturer’s bad post-inspection grade from the U.S. Food and Drug Administration – labeled an “Official Action Indicated” classification – is generally devastating for the facility, not least because it can stall FDA approval of applications to market drugs manufactured at the facility.

Pharmaceutical Technology
NOVEMBER 21, 2022
Japan is currently the fourth largest market in the world. Based on GlobalData estimates, the Japanese pharmaceutical market generated JPY9.392 trillion ($67.32 Other contributing factors include the decreasing number of post-market studies and difficulties in primary research and manufacturing.

STAT News
MARCH 30, 2023
What happened to the market for monkeys? Are some drugs too cheap? We also discuss what leads to generic drug shortages, whether every major pharmaceutical firm needs a weight-loss drug, and what it means when drug company cancels a conference appearance. And why are biotech stocks still in the tank?
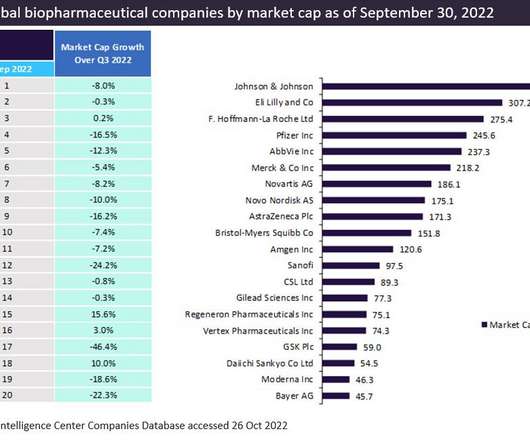
Pharmaceutical Technology
DECEMBER 20, 2022
The top 20 global biopharmaceutical companies exhibited a downward trend in aggregate market capitalisation by 9.1% This downturn in market cap was attributed to a decline in the demand for Covid-19 vaccines and therapies. trillion in Q2 2022 to $3.14 Eli Lilly was in second place, followed by Roche and Pfizer. and 10%, respectively.
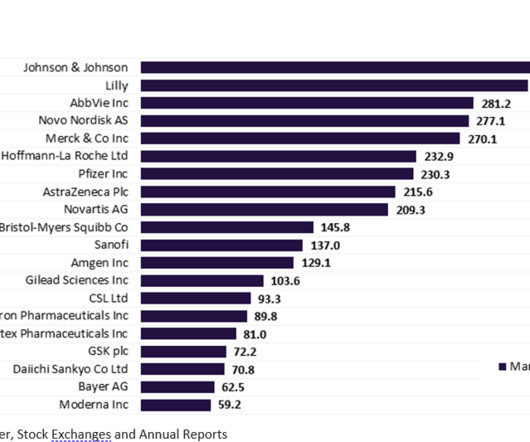
Pharmaceutical Technology
MAY 16, 2023
More than half of the top 20 global biopharmaceutical companies saw a fall in market capitalisation over Q1 2023. decline in total aggregate market capitalisation from $3.61 Bayer reported the highest market capitalisation growth of 23.1% Sanofi and Regeneron’s market capitalisation grew by 12.4% This resulted in a 3.4%
Pharmaceutical Commerce
FEBRUARY 9, 2024
Technavio analysis notes that an increase drug patent expirations could be a contributing factor.

Pharmaceutical Technology
SEPTEMBER 15, 2022
Various factors have contributed to the need and growth of API chemical suppliers such as rising healthcare expenditure, increasing disposable incomes, growing geriatric population, increasing incidence of chronic diseases, patent expiration of blockbuster drugs, increased consumption of generic drugs, and intervention of the new generation APIs.

Pharmaceutical Technology
JULY 25, 2022
Despite this, specialty generics are expected to be the domain of a handful of companies with the necessary manufacturing capabilities and legal backing needed for entering the market. Specialty drugs are most used to treat different cancers, rheumatoid arthritis, and multiple sclerosis. But there are some exceptions.

Pharmaceutical Technology
NOVEMBER 21, 2022
Japan is currently the fourth largest market in the world. Based on GlobalData estimates, the Japanese pharmaceutical market generated JPY9.392 trillion ($67.32 Other contributing factors include the decreasing number of post-market studies and difficulties in primary research and manufacturing.
Outsourcing Pharma
OCTOBER 12, 2020
Technology impacting generic drugs include artificial intelligence, telemedicine, fraud-preventing digital solutions and more, according to a report.

Fierce Pharma
NOVEMBER 22, 2023
Looking to protect its lucrative immunology drug Rinvoq, AbbVie is suing a clutch of generic drug makers that are attempting to market copycat versions of the blockbuster. |
Drug Patent Watch
JUNE 18, 2024
Branded generics are generic drugs that are marketed under a brand name by the manufacturer. Branded generics can be an attractive option for both consumers and pharmaceutical companies, offering cost savings while leveraging brand recognition. […] Source

FDA Law Blog
APRIL 2, 2024
Perhaps unsurprisingly given the extraordinary focus on drug pricing in the last decade, generic competition—FDA’s only real way to have an effect on drug pricing—tops this year’s list. FDA believes this change would effectuate timelier and more cost-efficient generic drug development.”

FDA Law Blog
NOVEMBER 16, 2021
5376 that has garnered the greatest amount of attention (at least in the food and drug law world) is TITLE XIII, Subtitle J (Drug Pricing), Part 1 (Lowering Prices Through Fair Drug Price Negotiation), Section 139001 (Providing for Lower Prices for Certain High-Priced Single Source Drugs). a) In General.—The
FDA Law Blog
OCTOBER 16, 2024
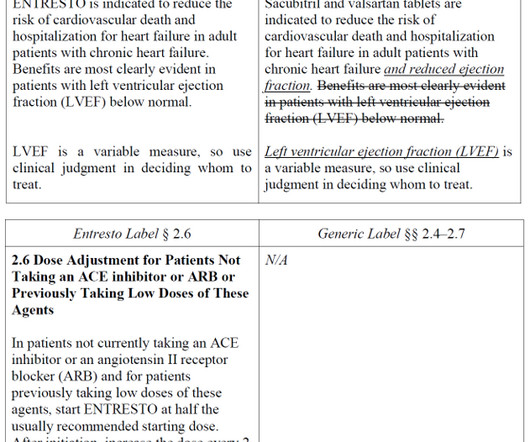
There, as we explained back in July , Novartis argued that FDA’s approval of a generic ENTRESTO with indication information modified rather than simply omitted “represents a sharp departure from FDA’s statutory and regulatory mandate to require that a generic drug be the ‘same’ as its reference listed drug.”
Pharmaceutical Technology
APRIL 4, 2023
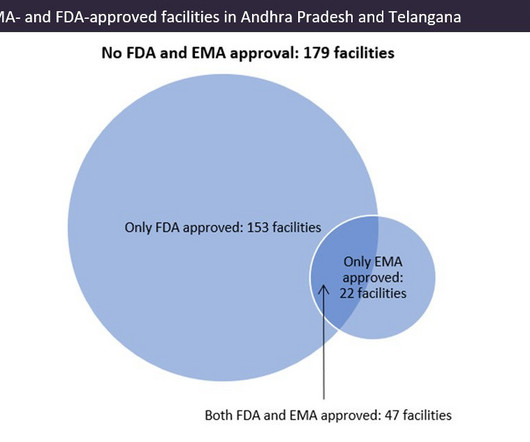
Indian pharma manufacturing continues to be the backbone of drug supplies worldwide, and GlobalData analysis suggests US overreliance on the country for generic drug supply. Source: GlobalData, Pharma Intelligence Center Drug Database (Accessed March 20, 2023). © GlobalData. ©GlobalData.

Pharmaceutical Technology
SEPTEMBER 23, 2022
For pharma and biotech companies, there are many advantages to partnering with a Contract Development and Manufacturing Organisations (CDMO): they can access more specialized knowledge, state-of-the-art equipment, and qualified staff, as well as reduce their total cost of ownership, all while maintaining speed to market.

World of DTC Marketing
APRIL 12, 2021
The goal of getting a drug to market as fast as possible so they can recoup drug development costs has the potential for mistakes that could cost lives. Coupled with all this is the continued outsourcing of raw materials and other steps in drug development. Up to 90% of drugs now sold in the US and UK are generic.

BioPharma Reporter
DECEMBER 22, 2022
Expanding generic drug competition and a decline in the demand for COVID-19 vaccines and therapies have brought down the aggregate market capitalization of the global top 20 biopharmaceutical companies by 9.1%.
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?


Let's personalize your content