Danaher to buy antibody supplier Abcam for $5.7B
Bio Pharma Dive
AUGUST 28, 2023
The acquisition gives Danaher ownership of a producer of research tools, such as antibodies and reagents, that are used in drug discovery experiments.
This site uses cookies to improve your experience. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country, we will assume you are from the United States. Select your Cookie Settings or view our Privacy Policy and Terms of Use.
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.
Used for the proper function of the website
Used for monitoring website traffic and interactions
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.

Worldwide Clinical Trials
SEPTEMBER 29, 2022
Preclinical assays designed to assess drug toxicity and safety must detect total drug. For studies of humanized therapeutics (monoclonal antibodies [mAb], fusion protein, antibody-drug conjugates [ADC], bispecific antibodies, etc.), a typical format is: receptor/target as capture with a generic binding reagent as detection (e.g.,
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

BioTech 365
AUGUST 9, 2021
Global Proteomics Market to 2026 – Analysis By Component (Instruments, Reagents, Services), & Application (Clinical Diagnostic, Drug Discovery, Others) – ResearchAndMarkets.com Global Proteomics Market to 2026 – Analysis By Component (Instruments, Reagents, Services), & Application (Clinical Diagnostic, Drug Discovery, Others) (..)

FDA Law Blog
SEPTEMBER 11, 2024
Claud — Last week, FDA revised one of its two guidances relating to nitrosamines, Control of Nitrosamine Impurities in Human Drugs. Nitrosamines are impurities that can form during drug manufacturing and are considered potentially potent carcinogens. It’s these varied scenarios that give quality managers nightmares.
Pharmaceutical Technology
FEBRUARY 15, 2023
“At the small scale, manufacturing between 1 ml and 10 ml is typically not problematic , but it’s not straightforward to replicate processes and technologies once you want to produce tens of litres of formulations,” says Dr Crowe, who manages a team that develop novel analytical assays related to LNPs and nanomaterials for drug delivery.

Camargo
JUNE 9, 2021
Drugs are delivered to where they need to go in the human body through any number of ROAs, by using orifices (such as oral or nasal delivery) or administering parenteral injections (such as intramuscular delivery). Intravesical administration involves introducing a urethral catheter to deliver a drug close to its target organ: the bladder.
XTalks
OCTOBER 7, 2020
With the need for COVID-19 testing growing amid the second wave of the pandemic, LabCorp (NYSE: LH) has received an emergency use authorization (EUA) from the US Food and Drug Administration (FDA) for its high-speed COVID-19 test. This will help reduce both the materials and time required to complete molecular COVID-19 tests.

Pharmaceutical Technology
SEPTEMBER 14, 2022
API peptides and proteins-based drugs have gained much attention in the past decade. Victoza, Copaxone, Lupron, Zoladex, Sandostatin, and Somatuline are some of the popularly marketed peptide API therapeutic drugs while more than 600 peptide-based pharmacological leads are being investigated worldwide, across various phases of development.

ProRelix Research
JUNE 26, 2023
As per Section 201(h)(1) of the Food, Drug, and Cosmetic Act, a device is an instrument, apparatus, implement, machine, contrivance, implant, in vitro reagent, or other similar or related article, […] The post US FDA Medical Device Applications appeared first on ProRelix Research.

The Pharma Data
NOVEMBER 30, 2020
The license from ERS Genomics now enables Vivlion to offer both R&D reagents and screening services to its customers worldwide. The license from ERS Genomics now enables Vivlion to offer both R&D reagents and screening services to its customers worldwide. CEO of Vivlion.
Roots Analysis
APRIL 5, 2023
Given the limitations of viral transfection as well as the apparent immunogenicity and cytotoxicity concerns associated with these vectors , demand for alternative gene drug vehicles is increasing. Currently, highest number of patent for non-viral transfection reagents and systems is filed in North America region.

FDA Law Blog
JANUARY 4, 2023
The purpose of these town halls are to discuss topics related to OTAT-regulated products, engage with product development stakeholders, and to provide information to help stakeholders to help advance drug development. The Agency also recommended not using substantial manipulations at a clinical site for already released final drug product.
XTalks
JANUARY 21, 2025
Agent drug-coated balloon. The balloons outer surface is coated with the drug paclitaxel, a safe and effective measure to prevent the arteries from narrowing again. The Onclarity HPV reagent pack extraction combo. Photo courtesy of Boston Scientific. Photo courtesy of BD.
XTalks
JULY 13, 2022
The Minuteful Kidney test is Healthy.io’s most recent innovation that has acquired 510(k) clearance from the US Food and Drug Administration (FDA). An ACR urine reagent dipstick/strip. An absorbent pad to remove excess liquid from the reagent strip. A color board that allows for image recognition.

Camargo
MAY 2, 2021
This innovation also continues to thrive across the pharmaceutical industry, as demonstrated by shifts in focus in oncology, rare diseases, and other areas of high unmet need that require new, more complex approaches to drug development. Identify critical development issues or deficiencies to address.

Pharmaceutical Technology
APRIL 28, 2023
Its scope includes Syngene providing integrated drug discovery and development solutions in discovery chemistry and biology, peptide chemistry, antibody and protein reagents, pharmacokinetics and drug metabolism, and pharmaceutical development. until 2026.

BioTech 365
SEPTEMBER 8, 2020
an industry pioneer in developing and commercializing diagnostic products and reagents for hematological malignancies, announced today key members of the leadership team hired to direct Invivoscribe Therapeutics, Inc., its fully integrated drug development engine. Invivoscribe Therapeutics … Continue reading →

XTalks
AUGUST 18, 2020
SalivaDirect, a saliva-based COVID-19 test that was tested among players of the National Basketball Association (NBA), has been given emergency use authorization (EUA) by the US Food and Drug Administration (FDA). The EUA comes after Yale partnered with the NBA to study the efficacy of the saliva-based test.

The Pharma Data
DECEMBER 2, 2020
Food and Drug Administration (FDA) for Onivyde (irinotecan liposome injection) for study patients with small cell lung cancer (SCLC) who progressed following a first-line platinum-based regimen. The drug is a small-molecule inhibitor of the S. Paris-based Ipsen secured Fast Track designation from the U.S. in Mainland China.

The Pharma Data
OCTOBER 27, 2020
Food and Drug Administration (FDA). ” With the panel’s authorization, Agena also aims to alleviate material shortages, enabling laboratories to accelerate testing without concerns about instrument or reagent availability. SAN DIEGO , Oct. ” More information is available at www.agenabio.com. About Agena Bioscience.

pharmaphorum
JANUARY 25, 2022
The Toronto-based company already counts many of the world’s largest pharma companies among its customers, using its platform for a range of tasks such as improving reagent and antibody selection to help scientists run more successful experiments, drawing on data from published studies and organisations’ internal databases.
pharmaphorum
FEBRUARY 3, 2021
While achieving the Nobel Prize spotlight would have been enough to impress, CRISPR-Cas9 gene editing is part of a growing list of technologies granted Investigational New Drug (IND) applications with early data from clinical trials supporting its safe use in edited cells re-introduced into a patient. Improving on impressive first steps.

Roots Analysis
FEBRUARY 21, 2022
Moreover, the manual protocols require extensive manipulation, costly reagents and long duration of skilled genomic library production. As a result, NGS library preparation kits have emerged as an innovative technology to overcome the challenges associated with conventional library preparation protocols. Nanoparticles Contract Manufacturing.

XTalks
APRIL 22, 2022
Both the areas of drug development and clinical trials are increasingly using in vitro assays to help determine the efficacy of an investigational therapeutic. Among these is the receptor occupancy assay, which is a powerful method used to understand how a therapeutic drug binds to (or occupies ) its receptors on the surface of certain cells.

The Pharma Data
AUGUST 19, 2021
Food and Drug Administration (FDA) approval of the VENTANA MMR RxDx Panel, advancing the company’s commitment to personalised healthcare through tests that determine which patients are most likely to benefit from specific and targeted therapies. today announced U.S. 4,5,6 PD-1 inhibitors can be effective in cancers with MMR deficiency.
The Pharma Data
MARCH 13, 2021
Takeda Pharmaceutical Company Limited ( TSE:4502/NYSE:TAK ) (“Takeda”) today announced that it has submitted a New Drug Application (NDA) to the Ministry of Health, Labour and Welfare (MHLW) in Japan for lanadelumab subcutaneous injection, a monoclonal antibody therapy for prophylaxis against attacks of hereditary angioedema (HAE).

pharmaphorum
SEPTEMBER 29, 2022
Food and Drug Administration (FDA) regulation as a medical device. Kyle helps companies build and refine corporate compliance programs, including advising clients on regulatory and compliance matters involving the Food, Drug and Cosmetic Act, the False Claims Act, the Anti-Kickback Statute, the AdvaMed Code and the PhRMA Code.

FDA Law Blog
AUGUST 3, 2022
Food and Drug Administration, Compliance Policy Guide, Commercialization of Unapproved In Vitro Diagnostic Devices Labeled for Research and Investigation (Aug. In the LDT’s “toddler years,” when issuing its analyte specific reagent rule in 1997, FDA stated that it would generally exercise enforcement discretion over LDTS.
Roots Analysis
FEBRUARY 13, 2023
The production of such drug products requires skilled labor, stringent manufacturing protocols and specialized expertise. Additionally, they also require appropriate drug delivery systems to efficiently administer the intervention (in a manner that they can avoid degradation by cellular endonucleases).

pharmaphorum
DECEMBER 16, 2022
Generative AI drug creation company Absci Corporation has added Dan Rabinovitsj (as pictured), VP of connectivity at Meta, to its Board of Directors. Optibrium, another software developer in the AI drug discovery space, has appointed Ian Smith as its new chief technology officer. AI and robotics hires. McKinnell, Jr.

The Pharma Data
MAY 4, 2021
The Philippine Food and Drug Administration (FDA) is seeking feedback on interim guidelines on the renewal of current good manufacturing practice (cGMP) clearance of foreign drug manufacturers. . Days after the media reports, CDSCO asked manufacturers and importers of tests and reagents to operate at maximum capacity.
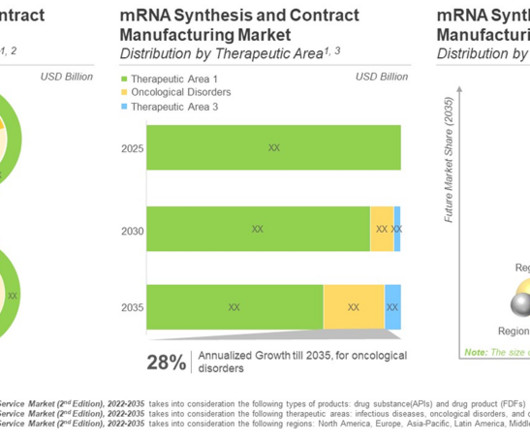
Roots Analysis
AUGUST 16, 2023
The success of COVID-19 vaccines paved the path for mRNA-based drug products. Market Landscape of mRNA Synthesis Kits Presently more than 95 mRNA synthesis kits, that contain reagents for the synthesis of research grade mRNAs with yield range up to 50,000 µg, are currently available in the market.

Roots Analysis
FEBRUARY 23, 2022
In the past two years, close to 3,220 patents related to NGS library preparation kits and reagents have been filed / granted, indicating the heightened pace of innovation. Next Generation Sequencing (NGS) Library Preparation Kits. Strong Intellectual Property Portfolio. Our Social Media Platform. Web: [link]. LinkedIn: [link].

The Pharma Data
JANUARY 24, 2021
“We are excited to welcome Chris, a highly regarded drug developer and team builder who brings to Notch great depth of experience and expertise in development of cell therapies, gene editing, and cell engineering spanning discovery through IND,” said David Main , President and Chief Executive Officer of Notch.

FDA Law Blog
NOVEMBER 1, 2023
Gibbs — For more than three decades, FDA has claimed that the Federal Food, Drug & Cosmetic (FD&C Act) gives the agency legal authority to regulate laboratory developed tests (LDTs) as medical devices (see our prior post here ). Shumsky & Philip Won & Adrienne R. Gaulkin & Jeffrey N.

FDA Law Blog
APRIL 19, 2021
FDA , the Court of Appeals ruled that FDA cannot regulate a medical product – in this case, the radiographic contrast agent barium sulfate – as a drug when the product meets the definition of a device. Circuit rejected FDA’s claim that it could regulate, at its discretion, any medical device as a drug. In Genus Medical Technologies v.

pharmaphorum
JUNE 1, 2021
The dramatic rise in online drug sales during the COVID-19 pandemic has increased the supply of counterfeit drugs, and many companies are now considering the addition of on-dose authentication for high-risk or high-value products. This had led to a corresponding rise in the supply of counterfeit drugs.

Roots Analysis
AUGUST 28, 2022
The growing interest of spatial omics solutions providers to diversify their products portfolio and enhance the abilities of their existing instruments, has led to several deals inked for the integration of spatial omics solutions with the contemporary staining assays / reagents. Our Social Media Platform. Web: [link].

The Pharma Data
OCTOBER 19, 2020
DB ( Becton, Dickinson and Company ) received a CE mark for its BD Multitest 6-Color TBNK Reagent with BD Trucount Tubes for assessing immune function in COVID-19 patients. A new clinical trial by the World Health Organization (WHO), however, reports that the drug does not have any particular effect on a patient’s survival.

XTalks
OCTOBER 19, 2023
An assay development scientist combines deep biological and biochemical expertise with practical laboratory skills to develop tests that can answer specific biological questions or drive the drug discovery process. This could involve tweaking experimental conditions, changing reagents or redesigning the assay.
Advarra
NOVEMBER 29, 2022
There have been recent signals indicating the Food and Drug Administration (FDA) plans to provide greater support for CGT, addressing key issues continuing to impede their development. No Prescription Drug User Fee Act (PDUFA) fee is associated with the INTERACT meetings. Recent Problems in Cell and Gene Therapy Development.

The Pharma Data
OCTOBER 14, 2020
They can be cultured to test drugs. The unique design of the system enables flowing through milliliters of blood samples within minutes, and completing an entire assay including reagent incubation steps within a few hours. “We expect our platform to be useful in a wide variety of conditions. Whole cells are precious biomarkers.

Advarra
OCTOBER 1, 2024
Regulatory costs: Regulatory costs may include submissions to regulatory authorities, such as applications for new investigational drug or device exemptions, annual reports, and final reports. from the Food and Drug Administration [FDA]) Safety reporting (e.g.,

FDA Law Blog
OCTOBER 6, 2023
The amended regulation would read as follows (revisions underlined): In vitro diagnostic products are those reagents, instruments, and systems intended for use in the diagnosis of disease or other conditions, including a determination of the state of health, in order to cure, mitigate, treat, or prevent disease or its sequelae.
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?



Let's personalize your content