FDA warns of rare hypersensitivity reaction due to antiseizure drugs
Pharmaceutical Technology
DECEMBER 29, 2023
FDA warns that levetiracetam and clobazam can cause DRESS, a rare hypersensitivity reaction which can be life threatening.

Pharmaceutical Technology
DECEMBER 29, 2023
FDA warns that levetiracetam and clobazam can cause DRESS, a rare hypersensitivity reaction which can be life threatening.
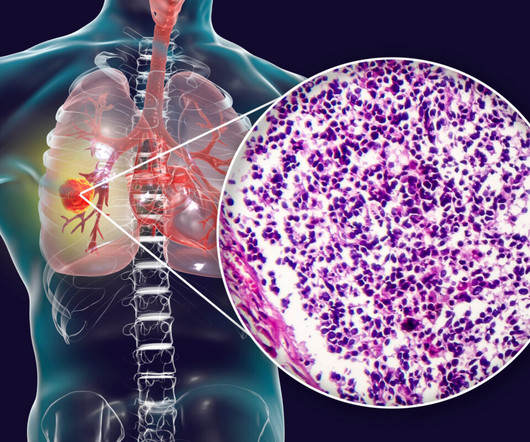
XTalks
DECEMBER 29, 2023
Amgen recently reported that the US Food and Drug Administration (FDA) has given priority review status to its drug candidate, tarlatamab, intended for the treatment of small-cell lung cancer (SCLC). If approved, tarlatamab could bring about significant improvements compared to existing options or potentially offer a treatment alternative in cases where no adequate therapy currently exists. “The FDA’s Priority Review designation for this application underscores the urgency to provide
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Pharmaceutical Technology
DECEMBER 29, 2023
Enveric Biosciences has selected EB-003 as its lead drug candidate from its EVM301 series for difficult-to-treat mental health disorders.

Fierce Pharma
DECEMBER 29, 2023
Armed with a new victory over Viatris in a patent dispute, Regeneron can knock off one contender in the Eylea biosimilar race. | A West Virginia judge found that Viatris' proposed biosimilar stepped on one of the three patents that Regeneron sued over. Shortly after, the company expanded its suit against another biosimilar contender, setting the stage for Regeneron's defense in Eylea's last few months of exclusivity.

Speaker: Simran Kaur, Founder & CEO at Tattva Health Inc.
The healthcare landscape is being revolutionized by AI and cutting-edge digital technologies, reshaping how patients receive care and interact with providers. In this webinar led by Simran Kaur, we will explore how AI-driven solutions are enhancing patient communication, improving care quality, and empowering preventive and predictive medicine. You'll also learn how AI is streamlining healthcare processes, helping providers offer more efficient, personalized care and enabling faster, data-driven
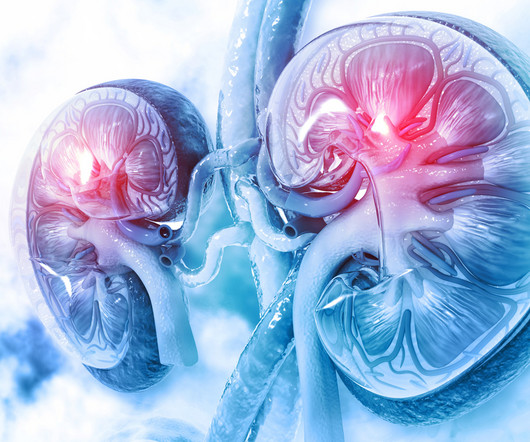
pharmaphorum
DECEMBER 29, 2023
Navigating transplant networks: Ensuring equitable access and improving outcomes for highly sensitised kidney transplant patients Mike.

Drug Patent Watch
DECEMBER 29, 2023
This chart shows the pharmaceutical companies with the most gel dosed drugs. For a different perspective, see the most popular dosage types. The companies with the most gel dosed drugs… The post Which pharmaceutical companies have the most gel dosed drugs? appeared first on DrugPatentWatch - Make Better Decisions.
Clinical Research Informer brings together the best content for clinical researchers from the widest variety of industry thought leaders.

Drug Patent Watch
DECEMBER 29, 2023
Annual Drug Patent Expirations for SAXENDA Saxenda is a drug marketed by Novo and is included in one NDA. It is available from one supplier. There are twenty-one patents protecting… The post New patent expiration for NOVO drug SAXENDA appeared first on DrugPatentWatch - Make Better Decisions.

pharmaphorum
DECEMBER 29, 2023
In this penultimate instalment of the five-part, festive series of the pharmaphorum podcast, host Nicole Raleigh speaks with Bobby Savarese, senior principal and business development director at Unispace Life Sciences, exploring how laboratory space requirements have changed since the pandemic.

Drug Patent Watch
DECEMBER 29, 2023
Annual Drug Patent Expirations for CHLORAPREP+WITH+TINT Chloraprep With Tint is a drug marketed by Becton Dickinson Co and is included in one NDA. It is available from two suppliers. There… The post New patent expiration for Becton Dickinson drug CHLORAPREP WITH TINT appeared first on DrugPatentWatch - Make Better Decisions.

Pharmaceutical Commerce
DECEMBER 29, 2023
Popular works in the category explore 340B guidelines, ‘smart pharma’ capabilities, and commercialization trends.

Drug Patent Watch
DECEMBER 29, 2023
Annual Drug Patent Expirations for CHLORAPREP+ONE-STEP Chloraprep One-step is a drug marketed by Becton Dickinson Co and is included in two NDAs. It is available from two suppliers. There are… The post New patent expiration for Becton Dickinson drug CHLORAPREP ONE-STEP appeared first on DrugPatentWatch - Make Better Decisions.

pharmaphorum
DECEMBER 29, 2023
To learn more, Deep Dive editor Eloise McLennan sat down with Calculus Capital’s investment director, Liz Klein, to discuss the dynamic world where capital meets compassion.

Drug Patent Watch
DECEMBER 29, 2023
Annual Drug Patent Expirations for VICTOZA Victoza is a drug marketed by Novo Nordisk Inc and is included in one NDA. It is available from two suppliers. There are six… The post New patent expiration for Novo Nordisk drug VICTOZA appeared first on DrugPatentWatch - Make Better Decisions.

Roots Analysis
DECEMBER 29, 2023
Clinical trials are prospective biomedical research studies designed to evaluate medical, surgical or behavioral interventions in people and investigate novel approaches for the diagnosis and prevention / treatment of diseases. In order to gain marketing approval from regulatory authorities for a novel therapeutic intervention, highly accurate and elaborate clinical trial data is required to validate the drug’s safety and effectiveness towards a specific target indication.

Advertisement
White paper that delves into the complex topic of Decentralized Clinical Trials and how to master them within the confines of FDA Regulations
Let's personalize your content