The future of cell and gene therapy manufacturing
Pharmaceutical Technology
NOVEMBER 30, 2023
As more cell and gene therapies get approved to treat common conditions, scaling them up will be a challenge.

Pharmaceutical Technology
NOVEMBER 30, 2023
As more cell and gene therapies get approved to treat common conditions, scaling them up will be a challenge.

Bio Pharma Dive
NOVEMBER 30, 2023
The biotech company creator’s last fund, which closed in 2021, brought in $3.4 billion for investing in new drug startups.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Pharmaceutical Technology
NOVEMBER 30, 2023
The move is a strategic response amid challenges faced by the non-viral gene therapy sector, with several startups closing this year.

Bio Pharma Dive
NOVEMBER 30, 2023
Trial contractor Fortrea is fighting back as Acelyrin unveils a CRO audit in the wake of a major late-stage study failure.

Speaker: Simran Kaur, Founder & CEO at Tattva Health Inc.
The healthcare landscape is being revolutionized by AI and cutting-edge digital technologies, reshaping how patients receive care and interact with providers. In this webinar led by Simran Kaur, we will explore how AI-driven solutions are enhancing patient communication, improving care quality, and empowering preventive and predictive medicine. You'll also learn how AI is streamlining healthcare processes, helping providers offer more efficient, personalized care and enabling faster, data-driven

Pharmaceutical Technology
NOVEMBER 30, 2023
GlobalData tracks 49 drugs in development for Gliosarcoma by 39 companies/universities/institutes. The top development phase for Gliosarcoma is phase i, with 22 drugs in that stage.

Bio Pharma Dive
NOVEMBER 30, 2023
The acquisition of ImmunoGen gives AbbVie an approved medicine for ovarian cancer, and continues a pharma spending spree on antibody-drug conjugates.
Clinical Research Informer brings together the best content for clinical researchers from the widest variety of industry thought leaders.

Rethinking Clinical Trials
NOVEMBER 30, 2023
The Health Care Systems Research Network (HCSRN) is accepting abstract submissions and panel presentation submissions for its 2024 Annual Conference until December 11, 2023. This year’s meeting will be held in Milwaukee, Wisconsin, from April 9 to 11, 2024. The HCSRN is a 20-member research network focused on supporting research institutes aligned with healthcare delivery systems.

Fierce Pharma
NOVEMBER 30, 2023
With layoffs hitting employees on both sides of the Atlantic, Pfizer’s $3.5 billion cost-cutting spree has kicked it into high gear this month. | The company's Groton, Connecticut, research site is the latest to fall victim to job cuts as part of Pfizer's massive $3.5 billion cost-cutting mission, following layoffs across the U.S. and the U.K.

Pharma Times
NOVEMBER 30, 2023
The new hub will unite researchers worldwide to exchange ideas and knowledge - News - PharmaTimes

Fierce Pharma
NOVEMBER 30, 2023
The sizzling antibody-drug conjugate (ADC) field is at the center of another major life sciences deal. | The sizzling antibody-drug conjugate field is at the center of another major life sciences deal. Hoping to redeem itself following the epic Rova-T failure, AbbVie is shelling out $10.1 billion in cash to acquire ImmunoGen, maker of the ovarian cancer treatment Elahere.

Pharmaceutical Technology
NOVEMBER 30, 2023
GlobalData tracks 15 drugs in development for Herpes Labialis (Oral Herpes) by 11 companies/universities/institutes. The top development phase for Herpes Labialis (Oral Herpes) is preclinical, with six drugs in that stage.

Pharma Mirror
NOVEMBER 30, 2023
By Alexei Voloshin, Global Head of Bioprocess Science, 3M Biopharmaceutical Purification Article Synopsis: Modern biotechnology has brought a revolution in improving the human condition around the world both in terms of quantity and quality. The rapid pace of innovation in biopharmaceutical treatments has enabled the treatment of life-threatening and life-changing conditions such as cancer, inflammation, and infectious disease.

Pharmaceutical Technology
NOVEMBER 30, 2023
Four non-stimulants are currently marketed in at least one of the 7MM: guanfacine, clonidine, atomoxetine and viloxazine.

Pharma Times
NOVEMBER 30, 2023
The new method successfully performs sterility assessments within 24 hours - News - PharmaTimes

Advertisement
White paper that delves into the complex topic of Decentralized Clinical Trials and how to master them within the confines of FDA Regulations

Pharmaceutical Technology
NOVEMBER 30, 2023
Since its implementation in 2022, new EU regulation for IVDs remains an obstacle for companies in the precision medicine sector.
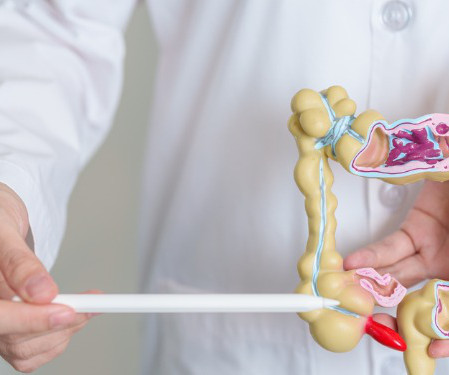
Antidote
NOVEMBER 30, 2023
Despite the fact that colorectal cancer cases have been declining in the United States since the mid-1980s, it is still the third most common cancer diagnosed each year excluding skin cancer. Often shortened to colon cancer, colorectal cancer occurs when cells in the colon and/or the rectum begin to grow uncontrollably and eventually spread to other parts of the body.

Pharmaceutical Technology
NOVEMBER 30, 2023
Culmination Bio has a library of electronic health records and biospecimen data that help in drug and diagnostic device development.

pharmaphorum
NOVEMBER 30, 2023
AbbVie has moved to shore up its pipeline following the loss of patent protection for its top-selling drug with a $10.1 billion deal to buy ImmunoGen and its marketed ovarian cancer drug.

Pharmaceutical Technology
NOVEMBER 30, 2023
Changing trends show a reduction in HCP contact with the pharma industry and an increased prioritisation of in-person contact.

pharmaphorum
NOVEMBER 30, 2023
FDA chief scientist Namandjé Bumpus has been named as principal deputy commissioner, replacing long-serving Janet Woodcock when she steps down early next year. Bumpus is heading for the number two position at the FDA – second only to Commissioner Robert Califf who announced the appointment on X (formerly Twitter) – just over a year after she joined the agency.

Pharmaceutical Technology
NOVEMBER 30, 2023
In the past few months, significant interest within the inflammatory bowel disease (IBD) space has been directed towards the therapeutic target, tumour necrosis factor-like cytokine 1A (TL1A).

XTalks
NOVEMBER 30, 2023
Patient-centric co-design refers to an approach where patients or end-users are actively involved with healthcare professionals, designers and other stakeholders to develop healthcare services or products. It allows for the creation of more effective, accessible and user-friendly healthcare solutions that align with the actual needs and desires of patients.

Advertisement
This new white paper defines and details the impact of Decentralized Clinical Trials on the Pharmaceutical industry and how the impact can be measured along with steps companies can take to ensure adoption.

Pharmaceutical Technology
NOVEMBER 30, 2023
Pepper’s Compass platform offers insight into the real-time function of proteins which can help in drug discovery.

XTalks
NOVEMBER 30, 2023
Tomorrow is World AIDS Day 2023. Every December 1, the global community comes together to observe World AIDS Day. Originating in 1988, this day serves as a crucial platform for increasing awareness about human immunodeficiency virus (HIV)/acquired immunodeficiency syndrome (AIDS) and paying tribute to those whose lives have been impacted by the epidemic.

Pharmaceutical Technology
NOVEMBER 30, 2023
MSD is one of many pharma companies migrating IT applications to the cloud to transform value chains and harness new technologies.

pharmaphorum
NOVEMBER 30, 2023
UK Biobank releases largest-ever genome sequencing dataset Phil.

Advertisement
Planning on running clinical trials in Japan? How can you reliably supply these studies? Discover Catalent’s clinical supply packaging facility in Shiga, Japan. Strategically located between Tokyo and Osaka, and one of largest in Japan, this 6,000 square meter facility offers comprehensive services including primary and secondary clinical packaging and labelling, comparator sourcing, cold chain storage, local and global distribution, local language support and white glove service to support stud

Pharmaceutical Technology
NOVEMBER 30, 2023
Experts at the clinical conference continued to explore ways to improve representation in clinical trials and the regulatory push behind it.

pharmaphorum
NOVEMBER 30, 2023
PCR in pharma packaging: A sustainable solution for tomorrow? Mike.

Intouch Solutions
NOVEMBER 30, 2023
Some EVERSANA INTOUCH colleagues around the globe observe and celebrate the national holiday of Thanksgiving. In our U.S.-based offices, we gathered for in-office potluck celebrations. During the gatherings, team members exchanged recipes, brought a dish to share with others, and, most importantly, spent time together! “The EVERSANA INTOUCH Friendsgiving potluck was a delight!

Fierce Pharma
NOVEMBER 30, 2023
Way back in 2015 a post on the Think with Google blog made the bold claim that, for marketers, consumer intent is more powerful than demographics: “Intent beats identity. | GoodRx is a trusted, high-intent platform that helps providers and patients find what they need at the exact moment they are looking for something. Learn why intent matters more than demographics.

Advertisement
Clinical trial data management is increasingly challenging as studies grow in complexity. Quickly accessing and analyzing study data is vital for assessing trial progress and patient safety. In this paper, we explore real-time data access and analysis for proactive study management. We investigate using adverse event (AE) data to monitor safety and discuss a clinical analytics platform that supports collaboration and data review workflows.
Let's personalize your content