Study suggests COVID-19 vaccination lowers incidence of arterial thromboses
Pharma Times
AUGUST 12, 2024
A second dose of the vaccine lowered the incidence of conditions such as heart attack or stroke
Pharma Times
AUGUST 12, 2024
A second dose of the vaccine lowered the incidence of conditions such as heart attack or stroke

Bio Pharma Dive
AUGUST 12, 2024
The clearance of Yorvipath for hypoparathyroidism was some time coming for Ascendis, which had resubmitted after receiving a rejection last year.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
Pharmaceutical Technology
AUGUST 12, 2024
As antibody drug conjugate (ADC)-centred deals dominate the oncology space, biotechs are using newer targets and linkers to differentiate themselves.

Bio Pharma Dive
AUGUST 12, 2024
Editors at Psychopharmacology cited "unethical conduct" that the study authors didn't disclose when submitting the papers. Lykos Therapeutics says it filed a complaint with a third party to review the way the journal came to its decision.

Speaker: Simran Kaur, Founder & CEO at Tattva Health Inc.
The healthcare landscape is being revolutionized by AI and cutting-edge digital technologies, reshaping how patients receive care and interact with providers. In this webinar led by Simran Kaur, we will explore how AI-driven solutions are enhancing patient communication, improving care quality, and empowering preventive and predictive medicine. You'll also learn how AI is streamlining healthcare processes, helping providers offer more efficient, personalized care and enabling faster, data-driven

Pharmaceutical Technology
AUGUST 12, 2024
The US FDA has approved ARS Pharmaceuticals' neffy, an anyphylaxis nasal spray 2mg, for the emergency treatment of allergic reactions.

Bio Pharma Dive
AUGUST 12, 2024
Results from a Phase 3 study could help Pfizer broaden use of its vaccine Abrysvo to younger adults whose medical history puts them at higher risk.
Clinical Research Informer brings together the best content for clinical researchers from the widest variety of industry thought leaders.

Bio Pharma Dive
AUGUST 12, 2024
Technology emerging from Yale researcher Craig Crews’ labs will be used to target prostate and breast cancer in early clinical trials.

Pharmaceutical Technology
AUGUST 12, 2024
Abrysvo was found to be safe in immunocompromised adults at risk for developing RSV-associated lower respiratory tract disease.

Fierce Pharma
AUGUST 12, 2024
Ascendis Pharma should be well prepared for the U.S. | After two delays, the FDA has finally signed off on Ascendis Pharma's hormone replacement therapy Yorvipath, also known as TransCon PTH, which is the first approved product for hypoparathyroidism in adults in the U.S.
Pharmaceutical Technology
AUGUST 12, 2024
As Regeneron and Bayer fend off competition for Eylea copycats in the courts, Sandoz says Enzeevu will be a “key biosimilar value driver.
Pharma Times
AUGUST 12, 2024
Bowel cancer was the most common cancer to be identified in both men and women
Pharmaceutical Technology
AUGUST 12, 2024
IASO Biotherapeutics has secured the US Food and Drug Administration (FDA) approval equecabtagene autoleucel (Eque-cel).

Fierce Pharma
AUGUST 12, 2024
Following last year’s acquisition of viral vector and plasmid specialist Forge Biologics, Japan’s Ajinomoto Bio-Pharma Services is streamlining its business in San Diego. | Following last year’s acquisition of viral vector and plasmid specialist Forge Biologics, Japan’s Ajinomoto Bio-Pharma Services is streamlining its business in San Diego. As a result, 71 California-based staffers—or 13% of the company’s overall workforce—are set to lose their jobs, Ajinomoto said in a memo shared with Fierce

Pharmaceutical Technology
AUGUST 12, 2024
Leap Consulting Group has launched a practice aimed at guiding Clinical Laboratory Improvement Amendments-certified labs (CLIA) in adhering to the US Food and Drug Administration’s (FDA) Final Rule over the classification of laboratory-developed tests (LDTs).

Advertisement
White paper that delves into the complex topic of Decentralized Clinical Trials and how to master them within the confines of FDA Regulations

pharmaphorum
AUGUST 12, 2024
Explore how the recent and upcoming elections may impact the field of life sciences and medical technology. Stay informed about the intersection of politics and health advancements.
Pharmaceutical Technology
AUGUST 12, 2024
IASO Biotherapeutics has secured the US Food and Drug Administration (FDA) approval equecabtagene autoleucel (Eque-cel).

pharmaphorum
AUGUST 12, 2024
MSD adds a bispecific antibody for blood cancers and autoimmune diseases to its pipeline via a $1.3bn licensing deal with Curon Biopharma.

Pharmaceutical Technology
AUGUST 12, 2024
The company plans to ask the US FDA to reconsider its request for an additional Phase III trial for the approval of its MDMA capsules.

Fierce Pharma
AUGUST 12, 2024
Locked in a closely watched respiratory syncytial virus (RSV) vaccine competition, Pfizer aims to expand the reach of its offering Abrysvo. | Locked in a closely watched RSV vaccine competition, Pfizer aims to expand the reach of its offering Abrysvo. Monday, Pfizer said Abrysvo elicited a strong response in adults with compromised immune systems.

Pharmaceutical Technology
AUGUST 12, 2024
Blue Earth Therapeutics' collaboration with Seibersdorf Labor will include the production of investigational 225Ac-based radioligand therapy.

Fierce Pharma
AUGUST 12, 2024
Despite hitting a roadblock on the path to approval for its once-weekly insulin icodec in the U.S., Novo Nordisk is continuing to move full speed ahead with a combo treatment pairing the drug with | Despite hitting a roadblock on the path to approval for its once-weekly insulin icodec in the United States, Novo Nordisk is continuing to move full speed ahead with a combo treatment pairing the drug with its unstoppable GLP-1 semaglutide.

Pharmaceutical Technology
AUGUST 12, 2024
Sable Therapeutics has signed an agreement with Columbia University to develop new polycation nanomedicines aimed at treating obesity.

Advertisement
This new white paper defines and details the impact of Decentralized Clinical Trials on the Pharmaceutical industry and how the impact can be measured along with steps companies can take to ensure adoption.

pharmaphorum
AUGUST 12, 2024
Journal retracts three articles on MDMA-assisted psychotherapy, shortly after FDA blocks approval of @Lykos_PBC's therapy for PTSD

XTalks
AUGUST 12, 2024
The US Food and Drug Administration (FDA) has approved ARS Pharmaceuticals’ neffy (epinephrine nasal spray) as the first non-needle emergency treatment of allergic reactions (Type I), including life-threatening ones like anaphylaxis, in adult and pediatric patients who weigh at least 66 pounds. Neffy is the first nasal spray formulation of epinephrine and is administrated as a single dose into one nostril.

pharmaphorum
AUGUST 12, 2024
Three controversial decisions by NICE on NHS use of new multiple myeloma therapies are heading for the end of comment and appeals processes.

Fierce Pharma
AUGUST 12, 2024
After treading the commercial path alone for the past year and change, Revance Therapeutics, maker of the Botox rival Daxxify, has agreed to fly the Crown Laboratories flag. | Revance and Crown Laboratories—a privately held skin care company with multiple marketed products of its own—are merging, the companies said in a release Monday. Under the deal, which has won the unanimous blessing of Revance’s board of directors, Crown will enter a tender offer to acquire all outstanding Revance stock for

Advertisement
Planning on running clinical trials in Japan? How can you reliably supply these studies? Discover Catalent’s clinical supply packaging facility in Shiga, Japan. Strategically located between Tokyo and Osaka, and one of largest in Japan, this 6,000 square meter facility offers comprehensive services including primary and secondary clinical packaging and labelling, comparator sourcing, cold chain storage, local and global distribution, local language support and white glove service to support stud

XTalks
AUGUST 12, 2024
The presence of lead in food remains a critical concern for the US Food and Drug Administration (FDA). Lead, a toxic metal, can enter the food supply through environmental contamination during the growing, raising or processing of foods. Even with the significant reduction of lead usage in industrial applications, residues from historical practices still pose a risk, necessitating stringent regulatory oversight.

Pharmaceutical Commerce
AUGUST 12, 2024
The latest news for pharma industry insiders.
pharmaphorum
AUGUST 12, 2024
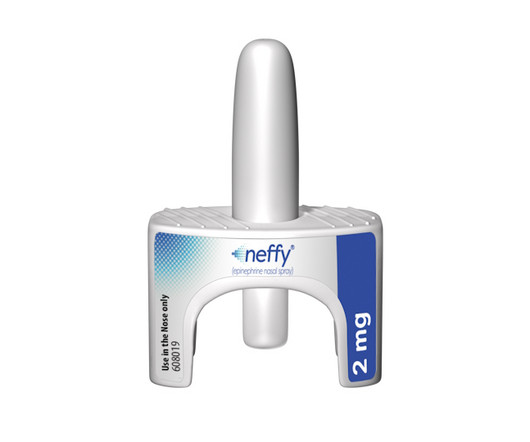
ARS Pharma gets FDA approval for epinephrine nasal spray neffy, the first needle-free alternative to EpiPen-style autoinjectors for serious allergic reactions.

Pharmaceutical Commerce
AUGUST 12, 2024
A lack of access could shut out patients in need of maintenance drugs for complex conditions.

Advertisement
Clinical trial data management is increasingly challenging as studies grow in complexity. Quickly accessing and analyzing study data is vital for assessing trial progress and patient safety. In this paper, we explore real-time data access and analysis for proactive study management. We investigate using adverse event (AE) data to monitor safety and discuss a clinical analytics platform that supports collaboration and data review workflows.
Let's personalize your content