Signal: Medtech’s Covid boom is well and truly over as Avail, Olive shutter
Pharmaceutical Technology
NOVEMBER 5, 2023
Two more medtech darlings have closed their doors this week, signalling an end to the funding rush of Covid.

Pharmaceutical Technology
NOVEMBER 5, 2023
Two more medtech darlings have closed their doors this week, signalling an end to the funding rush of Covid.

pharmaphorum
NOVEMBER 5, 2023
Innoviva’s ‘game changer’ antibiotic tackles gonorrhoea Phil.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Pharmaceutical Technology
NOVEMBER 5, 2023
KT-253 is under clinical development by Kymera Therapeutics and currently in Phase I for Myeloproliferative Disorders.

JAMA Internal Medicine
NOVEMBER 5, 2023
This French cohort study evaluates in-hospital mortality and adverse effects among older adults (≥75 years) who waited overnight in the emergency department for a bed in a hospital ward.

Speaker: Simran Kaur, Founder & CEO at Tattva Health Inc.
The healthcare landscape is being revolutionized by AI and cutting-edge digital technologies, reshaping how patients receive care and interact with providers. In this webinar led by Simran Kaur, we will explore how AI-driven solutions are enhancing patient communication, improving care quality, and empowering preventive and predictive medicine. You'll also learn how AI is streamlining healthcare processes, helping providers offer more efficient, personalized care and enabling faster, data-driven

Pharmaceutical Technology
NOVEMBER 5, 2023
ABP-671 is under clinical development by Jiangsu Atom Bioscience and Pharmaceutical and currently in Phase II for Hyperuricemia.

Drug Patent Watch
NOVEMBER 5, 2023
Annual Drug Patent Expirations for ACTONEL Actonel is a drug marketed by Apil and Warner Chilcott and is included in two NDAs. It is available from two suppliers. There are… The post New patent expiration for APIL drug ACTONEL appeared first on DrugPatentWatch - Make Better Decisions.
Clinical Research Informer brings together the best content for clinical researchers from the widest variety of industry thought leaders.

Pharmaceutical Technology
NOVEMBER 5, 2023
ALTO-100 is under clinical development by Alto Neuroscience and currently in Phase II for Post-Traumatic Stress Disorder (PTSD).

BioSpace
NOVEMBER 5, 2023
Seeking to weather declining sales from its COVID-19 business in the third quarter, Pfizer is laying off approximately 200 employees at its manufacturing facility in Kalamazoo, Michigan.

Pharmaceutical Technology
NOVEMBER 5, 2023
(Favezelimab + pembrolizumab) is under clinical development by Merck and currently in Phase II for Bladder Cancer.

BioSpace
NOVEMBER 5, 2023
After its deal with Tubulis in April 2023, Bristol Myers Squibb is continuing its antibody-drug conjugate buying spree by acquiring an asset from South Korea’s Orum Therapeutics.

Pharmaceutical Technology
NOVEMBER 5, 2023
(Favezelimab + pembrolizumab) is under clinical development by Merck and currently in Phase II for Melanoma.

JAMA Internal Medicine
NOVEMBER 5, 2023
This essay describes a palliative medicine physician’s experience with a dying patient and reflections on life.
Pharmaceutical Technology
NOVEMBER 5, 2023
KT-253 is under clinical development by Kymera Therapeutics and currently in Phase I for Acute Lymphocytic Leukemia (ALL, Acute Lymphoblastic Leukemia).

BioSpace
NOVEMBER 5, 2023
Although Kodiak Sciences initially scrapped its development of tarcocimab tedromer after late-stage failures, new data has convinced the company to give the eye drug another shot.

Advertisement
White paper that delves into the complex topic of Decentralized Clinical Trials and how to master them within the confines of FDA Regulations
Pharmaceutical Technology
NOVEMBER 5, 2023
KT-253 is under clinical development by Kymera Therapeutics and currently in Phase I for Lymphoma.

BioSpace
NOVEMBER 5, 2023
Navigating the Challenges of Clinical Trial Design - read this article along with other careers information, tips and advice on BioSpace

Pharmaceutical Technology
NOVEMBER 5, 2023
KT-253 is under clinical development by Kymera Therapeutics and currently in Phase I for Solid Tumor.

BioSpace
NOVEMBER 5, 2023
Following two late-stage failures, Travere Therapeutics has unveiled the results of two Phase III studies, attempting to regain Filspari’s footing in IgA nephropathy and focal segmental glomerulosclerosis.

Pharmaceutical Technology
NOVEMBER 5, 2023
KT-253 is under clinical development by Kymera Therapeutics and currently in Phase I for Myelodysplastic Syndrome.
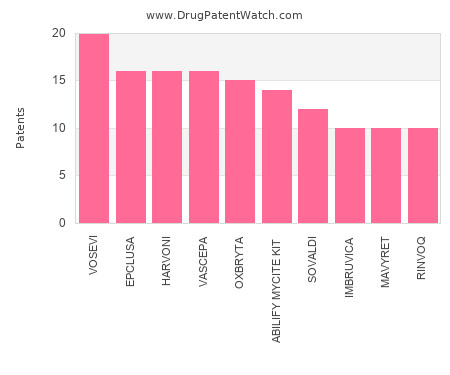
Drug Patent Watch
NOVEMBER 5, 2023
This chart shows the drugs with the most patents in Singapore. Patents must be filed in each country (or, in some cases regional patent office) where patent protection is desired.… The post Which pharmaceutical drugs have the most drug patents in Singapore? appeared first on DrugPatentWatch - Make Better Decisions.

Pharmaceutical Technology
NOVEMBER 5, 2023
KT-253 is under clinical development by Kymera Therapeutics and currently in Phase I for Refractory Acute Myeloid Leukemia.

Drug Patent Watch
NOVEMBER 5, 2023
Annual Drug Patent Expirations for ARMONAIR+RESPICLICK Armonair Respiclick is a drug marketed by Teva Pharm and is included in one NDA. It is available from one supplier. There are thirteen… The post New patent expiration for Teva Pharm drug ARMONAIR RESPICLICK appeared first on DrugPatentWatch - Make Better Decisions.

Advertisement
This new white paper defines and details the impact of Decentralized Clinical Trials on the Pharmaceutical industry and how the impact can be measured along with steps companies can take to ensure adoption.

Pharmaceutical Technology
NOVEMBER 5, 2023
KT-253 is under clinical development by Kymera Therapeutics and currently in Phase I for Myeloproliferative Disorders.

Drug Patent Watch
NOVEMBER 5, 2023
Annual Drug Patent Expirations for AIRDUO+DIGIHALER Airduo Digihaler is a drug marketed by Teva Pharm and is included in one NDA. It is available from four suppliers. There are twenty-eight… The post New patent expiration for Teva Pharm drug AIRDUO DIGIHALER appeared first on DrugPatentWatch - Make Better Decisions.

Pharmaceutical Technology
NOVEMBER 5, 2023
BGB-16673 is under clinical development by BeiGene and currently in Phase II for Waldenstrom Macroglobulinemia (Lymphoplasmacytic Lymphoma).

Drug Patent Watch
NOVEMBER 5, 2023
Annual Drug Patent Expirations for AIRDUO+RESPICLICK Airduo Respiclick is a drug marketed by Teva Pharm and is included in one NDA. It is available from four suppliers. There are sixteen… The post New patent expiration for Teva Pharm drug AIRDUO RESPICLICK appeared first on DrugPatentWatch - Make Better Decisions.

Advertisement
Planning on running clinical trials in Japan? How can you reliably supply these studies? Discover Catalent’s clinical supply packaging facility in Shiga, Japan. Strategically located between Tokyo and Osaka, and one of largest in Japan, this 6,000 square meter facility offers comprehensive services including primary and secondary clinical packaging and labelling, comparator sourcing, cold chain storage, local and global distribution, local language support and white glove service to support stud

Pharmaceutical Technology
NOVEMBER 5, 2023
ALTO-300 is under clinical development by Alto Neuroscience and currently in Phase II for Major Depressive Disorder.

Drug Patent Watch
NOVEMBER 5, 2023
Annual Drug Patent Expirations for ARMONAIR+DIGIHALER Armonair Digihaler is a drug marketed by Teva Pharm and is included in one NDA. It is available from one supplier. There are twenty-five… The post New patent expiration for Teva Pharm drug ARMONAIR DIGIHALER appeared first on DrugPatentWatch - Make Better Decisions.

Pharmaceutical Technology
NOVEMBER 5, 2023
ALTO-100 is under clinical development by Alto Neuroscience and currently in Phase II for Post-Traumatic Stress Disorder (PTSD).

FDA Law Blog
NOVEMBER 5, 2023
The 14th Asia Pacific Symposium on Cochlear Implant and Related Sciences is poised to captivate the global scientific community from November 8-11, 2023, as it convenes in Seoul, Korea. This prestigious four-day conference promises to deliver a wealth of cutting-edge insights, featuring presentations by eminent scientists and clinicians, all unified under the thought-provoking theme, “Towards Better Speech Perception and Beyond.

Advertisement
Clinical trial data management is increasingly challenging as studies grow in complexity. Quickly accessing and analyzing study data is vital for assessing trial progress and patient safety. In this paper, we explore real-time data access and analysis for proactive study management. We investigate using adverse event (AE) data to monitor safety and discuss a clinical analytics platform that supports collaboration and data review workflows.
Let's personalize your content