2023 in review: Cancer vaccines dose up on advances with tailored approaches
Pharmaceutical Technology
DECEMBER 22, 2023
As the number of patients with cancers continues to grow globally, research into personalised cancer vaccines is vital.
Pharmaceutical Technology
DECEMBER 22, 2023
As the number of patients with cancers continues to grow globally, research into personalised cancer vaccines is vital.

Bio Pharma Dive
DECEMBER 22, 2023
The deal would hand Bristol Myers, which has newly made neuroscience a therapeutic focus, an experimental treatment for schizophrenia.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Fierce Pharma
DECEMBER 22, 2023
Even with the Federal Trade Commission keeping a watchful eye on the biopharma industry and the economic landscape giving some players pause, mergers and acquisitions are back on the rise. | Even with the Federal Trade Commission keeping a watchful eye on the biopharma industry and the economic landscape giving some players pause, mergers and acquisitions are back on the rise.

Bio Pharma Dive
DECEMBER 22, 2023
The biotech is asking the agency to clear its treatment Elevidys in more patients with the disease, despite a confirmatory trial that missed its main goal.

Speaker: Simran Kaur, Founder & CEO at Tattva Health Inc.
The healthcare landscape is being revolutionized by AI and cutting-edge digital technologies, reshaping how patients receive care and interact with providers. In this webinar led by Simran Kaur, we will explore how AI-driven solutions are enhancing patient communication, improving care quality, and empowering preventive and predictive medicine. You'll also learn how AI is streamlining healthcare processes, helping providers offer more efficient, personalized care and enabling faster, data-driven

Pharmaceutical Technology
DECEMBER 22, 2023
Volrustomig is under clinical development by AstraZeneca and currently in Phase III for Cervical Cancer.

Bio Pharma Dive
DECEMBER 22, 2023
The clearance of Wainua for transthyretin amyloidosis opens up a new front in a long-running commercial battle between Ionis and Alnylam.
Clinical Research Informer brings together the best content for clinical researchers from the widest variety of industry thought leaders.

Fierce Pharma
DECEMBER 22, 2023
As the FDA investigates a safety signal for approved CAR-T therapies, the agency has upgraded a warning about secondary cancers resulting from use of Johnson & Johnson and Legend Biotech’s Carv | As the FDA investigates a safety signal for approved CAR-T therapies, the agency has elevated a warning about secondary cancers resulting from use of Johnson & Johnson and Legend Biotech’s Carvykti.

Pharmaceutical Technology
DECEMBER 22, 2023
Improve Vision with Implantable Corneal and Intraocular Implants - Patent Granted to Bausch Health Companies Inc. Discover a method for clear visualization of the eye using a photochromic polymer mask and controlled external stimuli.

Antidote
DECEMBER 22, 2023
Because Antidote’s key focus is connecting patients to clinical trials , hearing directly from individuals serves as a great reminder of why we do what we do. One patient we’ve had the pleasure of speaking with is Linda VandeVrede, a former high-tech PR executive who shared her story of chronic pain, clinical trials, and personal experience.

Pharmaceutical Technology
DECEMBER 22, 2023
Angelini Spa has filed a patent for a method and apparatus to produce fluff-free absorbent cores for sanitary articles. Discover how loose fibers and superabsorbent granular material are mixed and bound together to create an absorbent structure.

pharmaphorum
DECEMBER 22, 2023
This year brought about a whole gambit of news and developments, of conferences and launches, all coming in thick and fast and excited about the pharmaceutical industry and healthcare system. Not least of these was Smith + Nephew’s opening of a new state-of-the-art surgical innovation and training centre in the heart of Munich, Germany, on 11th October.

Pharmaceutical Technology
DECEMBER 22, 2023
This signifies the third submission in four months from J&J for the Rybrevant development programme.

Fierce Pharma
DECEMBER 22, 2023
The FDA and Novo Nordisk are warning of c | The FDA and Novo Nordisk are warning of counterfeit versions of popular weight-loss treatment Ozempic that have been discovered in the “legitimate” United States supply chain.

Pharmaceutical Technology
DECEMBER 22, 2023
ImmunoScape has signed a collaboration with EDDC to develop new T cell receptor (TCR)-based bispecific molecules for solid tumours.

Advertisement
White paper that delves into the complex topic of Decentralized Clinical Trials and how to master them within the confines of FDA Regulations

pharmaphorum
DECEMBER 22, 2023
AstraZeneca and Ionis get first approval for ATTR polyneuropathy drug Wainua (eplontersen), a rival to drugs from Alnylam and Pfizer

Pharmaceutical Technology
DECEMBER 22, 2023
Ono has signed an agreement with EVQLV to utilise its AI-powered antibody design engine for the development of new antibody drugs.

XTalks
DECEMBER 22, 2023
Glaukos Corporation, a leader in ophthalmic medical technology and pharmaceuticals with a focus on novel treatments for glaucoma, corneal disorders and retinal diseases, has secured US Food and Drug Administration (FDA) approval for iDose TR (travoprost intracameral implant) 75 mcg, a prostaglandin analog intended for one-time use per eye. This innovative therapy is aimed at decreasing intraocular pressure (IOP) in individuals with ocular hypertension (OHT) or open-angle glaucoma (OAG).

Pharmaceutical Technology
DECEMBER 22, 2023
The China NMPA approved Takeda’s Livtencity to treat adults with post-HSCT or solid organ transplant cytomegalovirus infection/disease.

pharmaphorum
DECEMBER 22, 2023
Discover three key strategies to make 2024 the year of the patient in healthcare. Learn how revolutionising trial design, embracing technology, and breaking down silos can improve patient care and outcomes.

Pharmaceutical Technology
DECEMBER 22, 2023
The US FDA has granted breakthrough therapy designation for BioNTech and DualityBio's BNT323/DB-1303 to treat advanced endometrial cancer.

pharmaphorum
DECEMBER 22, 2023
Discover how AI is transforming the healthcare industry by automating boring and mundane tasks, allowing healthcare professionals to focus on more interesting and important aspects of patient care.

Pharmaceutical Technology
DECEMBER 22, 2023
IBI-322 is under clinical development by Innovent Biologics and currently in Phase I for Lymphoma.

Advertisement
This new white paper defines and details the impact of Decentralized Clinical Trials on the Pharmaceutical industry and how the impact can be measured along with steps companies can take to ensure adoption.
Drug Patent Watch
DECEMBER 22, 2023
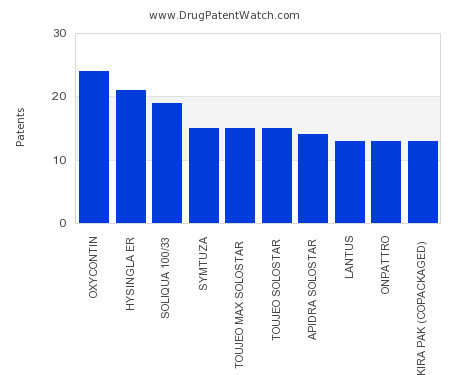
This chart shows the drugs with the most patents in Cyprus. Patents must be filed in each country (or, in some cases regional patent office) where patent protection is desired.… The post Which pharmaceutical drugs have the most drug patents in Cyprus? appeared first on DrugPatentWatch - Make Better Decisions.

Pharmaceutical Technology
DECEMBER 22, 2023
Adagrasib is under clinical development by Bristol-Myers Squibb and currently in Phase III for Squamous Non-Small Cell Lung Cancer.

Drug Patent Watch
DECEMBER 22, 2023
Annual Drug Patent Expirations for NEXLIZET Nexlizet is a drug marketed by Esperion Theraps Inc and is included in one NDA. It is available from one supplier. There are ten… The post New patent expiration for Esperion Theraps drug NEXLIZET appeared first on DrugPatentWatch - Make Better Decisions.

Pharmaceutical Technology
DECEMBER 22, 2023
SOR-007 is under clinical development by Nanology and currently in Phase II for Metastatic Adenocarcinoma of The Pancreas.

Advertisement
Planning on running clinical trials in Japan? How can you reliably supply these studies? Discover Catalent’s clinical supply packaging facility in Shiga, Japan. Strategically located between Tokyo and Osaka, and one of largest in Japan, this 6,000 square meter facility offers comprehensive services including primary and secondary clinical packaging and labelling, comparator sourcing, cold chain storage, local and global distribution, local language support and white glove service to support stud

Drug Patent Watch
DECEMBER 22, 2023
Annual Drug Patent Expirations for FINTEPLA Fintepla is a drug marketed by Ucb Inc and is included in one NDA. It is available from one supplier. There are twelve patents… The post New patent for Ucb Inc drug FINTEPLA appeared first on DrugPatentWatch - Make Better Decisions.

Pharmaceutical Technology
DECEMBER 22, 2023
Cadonilimab is under clinical development by Akeso and currently in Phase II for Esophageal Squamous Cell Carcinoma (ESCC).

Drug Patent Watch
DECEMBER 22, 2023
Annual Drug Patent Expirations for AUSTEDO+XR Austedo Xr is a drug marketed by Teva and is included in one NDA. It is available from one supplier. There are nine patents… The post New patent for TEVA drug AUSTEDO XR appeared first on DrugPatentWatch - Make Better Decisions.

Pharmaceutical Technology
DECEMBER 22, 2023
MRNA-2752 is under clinical development by Moderna and currently in Phase I for Transitional Cell Cancer (Urothelial Cell Cancer).

Advertisement
Clinical trial data management is increasingly challenging as studies grow in complexity. Quickly accessing and analyzing study data is vital for assessing trial progress and patient safety. In this paper, we explore real-time data access and analysis for proactive study management. We investigate using adverse event (AE) data to monitor safety and discuss a clinical analytics platform that supports collaboration and data review workflows.
Let's personalize your content