Will the tide turn for the wave of pharma layoffs?
Pharmaceutical Technology
OCTOBER 30, 2024
Pharmaceutical management expert Kenneth Getz shares insights on broader trends in the pharma sector influencing the recent wave of layoffs.

Pharmaceutical Technology
OCTOBER 30, 2024
Pharmaceutical management expert Kenneth Getz shares insights on broader trends in the pharma sector influencing the recent wave of layoffs.

Bio Pharma Dive
OCTOBER 30, 2024
Archon Biosciences, co-founded by the winner of the 2024 Nobel Prize in Chemistry, emerged from stealth Wednesday with $20 million in seed capital.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Rethinking Clinical Trials
OCTOBER 30, 2024
Dr. Thomas Carton and Dr. Anitha John In this Friday’s PCT Grand Rounds, Thomas Carton of the Louisiana Public Health Institute and Anitha John of George Washington University and the Children’s National Hospital will present “Congenital Heart Initiative: Redefining Outcomes and Navigation to Adult Centered Care (CHI-RON) Study.” The Grand Rounds session will be held on Friday, November 1, 2024, at 1:00 pm eastern.

Bio Pharma Dive
OCTOBER 30, 2024
The partners no longer intend to develop Zurzuvae for one of the most common forms of depression, citing the time and cost of running new studies that would satisfy the FDA.

Speaker: Simran Kaur, Co-founder & CEO at Tattva Health Inc.
AI is transforming clinical trials—accelerating drug discovery, optimizing patient recruitment, and improving data analysis. But its impact goes far beyond research. As AI-driven innovation reshapes the clinical trial process, it’s also influencing broader healthcare trends, from personalized medicine to patient outcomes. Join this new webinar featuring Simran Kaur for an insightful discussion on what all of this means for the future of healthcare!
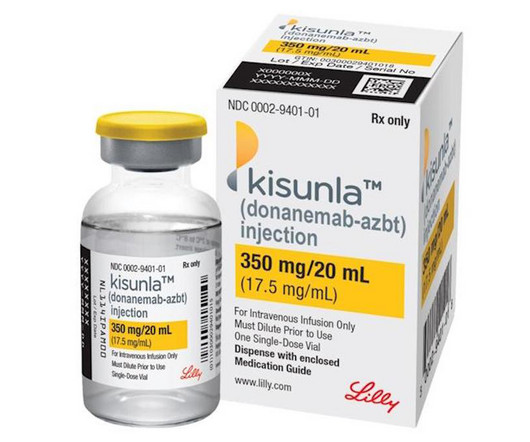
pharmaphorum
OCTOBER 30, 2024
Eli Lilly says a new starting dose regimen for its Alzheimer's disease therapy Kisunla reduces the risk of side effects that have stood in the way of approvals and reimbursement decisions for drugs in its class.Simply shifting one vial of the anti-amyloid antibody from the first infusion to the third infusion of the titration phase of dosing – the period used to achieve therapeutic levels in the body – is enough to lower the incidence of a potentially serious side effect known as ARIA-E, accordi

Bio Pharma Dive
OCTOBER 30, 2024
Third quarter Mounjaro and Zepbound sales, while up substantially versus the same period last year, grew little compared to the second quarter amid inventory decreases in the U.S.
Clinical Research Informer brings together the best content for clinical researchers from the widest variety of industry thought leaders.

Bio Pharma Dive
OCTOBER 30, 2024
The British pharma’s experience during the third quarter mirrored that of rival Pfizer, which also reported lower sales of its competing RSV shot.

Pharmaceutical Technology
OCTOBER 30, 2024
GlaxoSmithKline (GSK) has entered an agreement to acquire Chimagen Biosciences’ CMG1A46 for $300m upfront.

Bio Pharma Dive
OCTOBER 30, 2024
All doses of the two drugs are now listed as available in the U.S., per an FDA database, although the company cautioned patients may still experience "variability" filling their prescriptions.

Pharmaceutical Technology
OCTOBER 30, 2024
Green chemistry has the potential to revolutionise the pharmaceutical industry.

Speaker: Simran Kaur, Founder & CEO at Tattva Health Inc.
The healthcare landscape is being revolutionized by AI and cutting-edge digital technologies, reshaping how patients receive care and interact with providers. In this webinar led by Simran Kaur, we will explore how AI-driven solutions are enhancing patient communication, improving care quality, and empowering preventive and predictive medicine. You'll also learn how AI is streamlining healthcare processes, helping providers offer more efficient, personalized care and enabling faster, data-driven

Bio Pharma Dive
OCTOBER 30, 2024
The funding for Axonis, which is developing novel medicines for pain and epilepsy, reflects growing confidence in precision therapies for neurological diseases, one investor told BioPharma Dive.

ACRP blog
OCTOBER 30, 2024
In 1976, at a moment in history when U.S. enrollment of women in medical schools had just climbed to 20%, it is notable that the chronicles of the Association of Clinical Research Professionals (ACRP) describe the first meeting of two nurses who were among the field’s earliest clinical research coordinators, Sarah Boyer and Anne LeSher, as the impetus for the formation of the Association.

Bio Pharma Dive
OCTOBER 30, 2024
Even though Biogen raised earnings guidance, the company still has “some potentially concerning signals” in its base business, according to one analyst.

Pharmaceutical Technology
OCTOBER 30, 2024
Barman-Aksӧzen shared her experience of guiding treatment research for her own ultra rare disorder, erythropoietic protoporphyria (EPP).

pharmaphorum
OCTOBER 30, 2024
Novartis says FDA approval for frontline use of its leukaemia drug Scemblix will boost the number of eligible patients four-fold, as it hunts blockbuster sales
Pharmaceutical Technology
OCTOBER 30, 2024
Bladder cancer is the ninth most common cancer globally, and the number of diagnosed incident cases is set to increase.

pharmaphorum
OCTOBER 30, 2024
Discover how GenAI is leveraging AI technology to streamline R&D processes, ensuring trusted data and accelerating drug discovery timelines while reducing costs.

Advertisement
White paper that delves into the complex topic of Decentralized Clinical Trials and how to master them within the confines of FDA Regulations

Pharmaceutical Technology
OCTOBER 30, 2024
With the formation of a new strategic partnership, Dyno Therapeutics is responsible for the development of novel AAV capsids.

pharmaphorum
OCTOBER 30, 2024
The UK's first female Chancellor Rachel Reeves pumps billions into NHS in the first Labour budget in 14 years

Pharmaceutical Technology
OCTOBER 30, 2024
Amidst a share value slump and executive turmoil, Pfizer reported a robust quarter thanks in part to Covid-19 drug sales.

pharmaphorum
OCTOBER 30, 2024
The head of AstraZeneca's Chinese operations, Leon Wang, has been placed under investigation by the authorities in Beijing
Pharmaceutical Technology
OCTOBER 30, 2024
The company has laid off 39% of its staff as it discontinued the development of SPR720 for non-tuberculous mycobacterial pulmonary disease.

pharmaphorum
OCTOBER 30, 2024
Discover how leveraging AI can help biopharma companies future-proof their operations and strategies. Learn about innovative AI strategies that can drive success in the biopharma industry.

Pharmaceutical Technology
OCTOBER 30, 2024
Lilly’s Alzheimer’s drug Kisunla was approved in the US, Japan, and the UK, among other countries.

pharmaphorum
OCTOBER 30, 2024
The recent FDA decision on the use of MDMA for PTSD treatment has created uncertainty about the future of psychedelic therapy. Learn more about the implications and potential impact on patients.

Advertisement
This new white paper defines and details the impact of Decentralized Clinical Trials on the Pharmaceutical industry and how the impact can be measured along with steps companies can take to ensure adoption.

Pharmaceutical Technology
OCTOBER 30, 2024
Novartis has received FDA accelerated approval for Scemblix (asciminib) to treat adults with newly diagnosed Ph+ CML-CP.
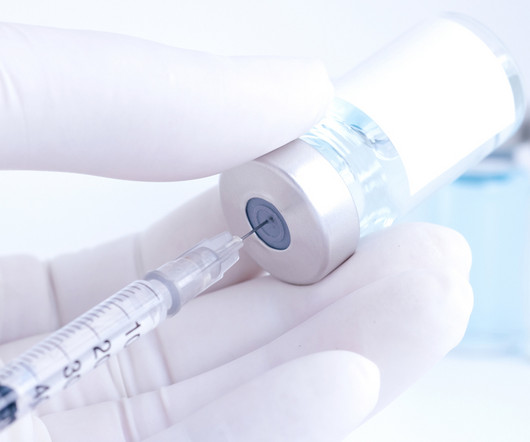
pharmaphorum
OCTOBER 30, 2024
Learn how personalised cancer vaccines are revolutionising cancer treatment and how regulatory bodies like the FDA juggle trade-offs to ensure safe and effective treatments.

Pharmaceutical Technology
OCTOBER 30, 2024
The Kalihinol analogue is an innovative approach to malaria treatment, targeting the apicoplast to undermine P falciparum’s functions.

Fierce Pharma
OCTOBER 30, 2024
Baxter International plans to exit China’s intravenous fluids market, multiple local media outlets report, citing a letter to distributors. | Baxter plans to exit China's IV fluids market as it faces tough local competition and as it redirects global IV supplies to the U.S. to cope with a post-hurricane shortage.

Advertisement
Planning on running clinical trials in Japan? How can you reliably supply these studies? Discover Catalent’s clinical supply packaging facility in Shiga, Japan. Strategically located between Tokyo and Osaka, and one of largest in Japan, this 6,000 square meter facility offers comprehensive services including primary and secondary clinical packaging and labelling, comparator sourcing, cold chain storage, local and global distribution, local language support and white glove service to support stud
Let's personalize your content