Character Biosciences raises $93M with an eye on vision loss drugs
Bio Pharma Dive
MARCH 25, 2025
The startup is bringing into Phase 1 testing an experimental treatment it says could be more effective than marketed geographic atrophy medicines.

Bio Pharma Dive
MARCH 25, 2025
The startup is bringing into Phase 1 testing an experimental treatment it says could be more effective than marketed geographic atrophy medicines.

Pharmaceutical Technology
MARCH 25, 2025
The study will analyse electronic health records of 65- and 66-year olds from the UKs National Health Service (NHS).
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Bio Pharma Dive
MARCH 25, 2025
A licensing deal with Jiangsu Hengrui Pharmaceuticals puts Merck in a competitive race to develop a medicine that targets a genetic risk factor called lipoprotein(a).

Pharmaceutical Technology
MARCH 25, 2025
WuXi AppTec (Shanghai, China) has sold off two US/UK businesses in February and March, it revealed in its annual results on 18 March 2025.

Speaker: Simran Kaur, Co-founder & CEO at Tattva.Health
AI is transforming clinical trials—accelerating drug discovery, optimizing patient recruitment, and improving data analysis. But its impact goes far beyond research. As AI-driven innovation reshapes the clinical trial process, it’s also influencing broader healthcare trends, from personalized medicine to patient outcomes. Join this new webinar featuring Simran Kaur for an insightful discussion on what all of this means for the future of healthcare!

FDA Law Blog
MARCH 25, 2025
By Adrienne R. Lenz, Principal Medical Device Regulation Expert Last year, FDA issued a letter to the medical device industry warning medical device firms of concerns related to fraudulent and unreliable laboratory testing data in premarket submissions, which we blogged about here. This was followed last September with warning letters issued to two Chinese firms performing biocompatibility testing, citing violations of 21 C.F.R.

Pharmaceutical Technology
MARCH 25, 2025
Novo Nordisk has acquired the global rights to United Laboratories triple-agonist weight-loss and diabetes drug in a deal worth up to $2bn.
Clinical Research Informer brings together the best content for clinical researchers from the widest variety of industry thought leaders.

Pharmaceutical Technology
MARCH 25, 2025
Evolving regulations in sterile manufacturing requires innovation, market knowledge, and investment. How can drug manufacturers respond?
pharmaphorum
MARCH 25, 2025
Explore the latest M&A opportunities in the UK weight loss market, including market access for products like Wegovy and Mounjaro. Stay informed about key trends and opportunities in the industry.

Pharmaceutical Technology
MARCH 25, 2025
Alumis and Kaken Pharmaceutical have entered a partnership and licensing agreement for the development of the formers ESK-001.

pharmaphorum
MARCH 25, 2025
GSK joins with two UK research organisations to explore a possible link between its shingles vaccine Shingrix and a reduced risk of dementia.

Speaker: Simran Kaur, Founder & CEO at Tattva Health Inc.
The healthcare landscape is being revolutionized by AI and cutting-edge digital technologies, reshaping how patients receive care and interact with providers. In this webinar led by Simran Kaur, we will explore how AI-driven solutions are enhancing patient communication, improving care quality, and empowering preventive and predictive medicine. You'll also learn how AI is streamlining healthcare processes, helping providers offer more efficient, personalized care and enabling faster, data-driven

Pharmaceutical Technology
MARCH 25, 2025
The EMA has agreed to review GSKs application to expand Nucalas use as an add-on maintenance for individuals with COPD.

pharmaphorum
MARCH 25, 2025
A multibillion-dollar partnership between AbbVie and Genmab risks being soured by a lawsuit claiming misappropriation of trade secrets

Pharmaceutical Technology
MARCH 25, 2025
The agency has published a list of 24 high-risk infectious diseases as a reference tool for funders and researchers.

Pharmaceutical Commerce
MARCH 25, 2025
What drivers impact the differences in expenditures?

Pharmaceutical Technology
MARCH 25, 2025
Sermonix Pharmaceuticals and Regor have announced a strategic partnership aimed at developing therapeutics in the breast oncology area.

Pharmaceutical Commerce
MARCH 25, 2025
The latest news for pharma industry insiders.

pharmaphorum
MARCH 25, 2025
MSD has fleshed out its cardiovascular disease pipeline by licensing an oral Lp(a) inhibitor from China's Jiangsu Hengrui Pharma for $200m upfront
AuroBlog - Aurous Healthcare Clinical Trials blog
MARCH 25, 2025
Strokes are caused by a blockage in the brain’s blood vessels. (piyaset/iStock/Getty Images Plus) As a nurse working in a neurocritical care, I witnessed the sudden and devastating effects of stroke on survivors and their carers. Following my nursing career, I became a researcher specializing in stroke.

Advertisement
White paper that delves into the complex topic of Decentralized Clinical Trials and how to master them within the confines of FDA Regulations

pharmaphorum
MARCH 25, 2025
EVERSANA discuss 5 effective strategies for overcoming pricing and access challenges in the European healthcare landscape. Learn about HTA, risk-sharing, data utilisation, orphan drugs, and more.

AuroBlog - Aurous Healthcare Clinical Trials blog
MARCH 25, 2025
Exports of Ayush and herbal products for the first nine months of the current fiscal have reported a 9.9 per cent growth, while the third quarter of the fiscal reported growth of 9 per cent compared to the same period of previous fiscal year.
Pharma Mirror
MARCH 25, 2025
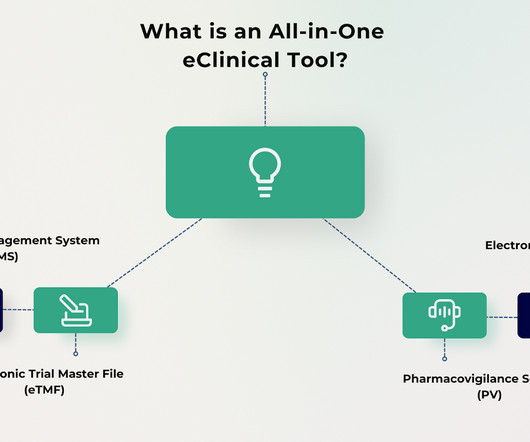
Clinical trials have grown increasingly complex and now require robust solutions to manage data, documentation, and workflows. Traditionally, organizations have relied on separate systems for Clinical Trial Management Systems (CTMS) and Electronic Trial Master Files (eTMF), often leading to inefficiencies, compliance risks, and administrative burdens.

Pharma Marketing Network
MARCH 25, 2025
Automation, AI, budget cuts, and evolving digital strategies have prompted a growing question among pharmaceutical marketing teams: Is my pharma marketing job at risk? As companies adapt to more agile workflows and redefine what modern commercialization looks like, the roles of marketing professionals are being evaluated more critically than ever before.

XTalks
MARCH 25, 2025
The development of mRNA vaccines has revolutionized the field of immunization, offering a rapid, adaptable and effective approach to combating infectious diseases. However, ensuring their safety, efficacy and stability requires precise characterization throughout the development and manufacturing process. Characterizing mRNA vaccines involves evaluating molecular integrity, purity, potency and immunogenicity, as well as ensuring consistent large-scale production.

Pharma Marketing Network
MARCH 25, 2025
Healthcare professionals (HCPs) are the cornerstone of pharmaceutical success, yet reaching them has never been more complex. Between digital saturation, regulatory scrutiny, and shifting engagement preferences, marketers must be both strategic and adaptable. Understanding how to effectively engage HCPs in pharma marketing is essential for driving brand awareness, product education, and ultimately, prescription behavior.

Imperical Blog
MARCH 25, 2025
Creating materials for pediatric participants, whether in digital or printed formats, presents unique challenges. This is due to the need to communicate complex ideas in simpler terms as well as create documents that appeal to that audience. Read on for tips to engage pediatric participants… The post How Do You Engage Pediatric Participants When Developing Clinical Trial Materials?

XTalks
MARCH 25, 2025
Clinical trials in the US are undergoing rapid transformation in 2025. Driven by advances in digital technology, regulatory updates and growing emphasis on patient-centric design, sponsors and research organizations are rethinking how studies are designed, conducted and analyzed. From the rise of decentralized trial models and wearable technology to the integration of real-world evidence and AI-powered data management, these trends are reshaping timelines, accessibility and efficiency.

Advertisement
This new white paper defines and details the impact of Decentralized Clinical Trials on the Pharmaceutical industry and how the impact can be measured along with steps companies can take to ensure adoption.

Drug Patent Watch
MARCH 25, 2025
Breaking into the Generic Drug Market: Strategies for Success As a pharmaceutical professional, you know that the generic drug market is a highly competitive space. With new generic drugs entering the market every year, it can be challenging to stand out from the crowd and achieve success. But what sets the successful generic drug companies apart from the rest?

Drug Channels
MARCH 25, 2025
Wow! What an absolutely incredible time at the inaugural Drug Channels Leadership Forum (DCLF)! Paula and I are beyond grateful to everyone who took the stage to share their insights and to all who participated in making this event so impactful. (Even Paula got on the main stage!) The event was packed with thought-provoking discussions, candid insights, and dynamic exchanges.

My Local Study
MARCH 25, 2025
March is Endometriosis Awareness Month, a time to bring attention to a condition that affects an estimated 1 in 10 women of reproductive age worldwide. Endometriosis. The word itself can feel heavy, echoing the weight of a condition often shrouded in silence, misunderstood, and frequently dismissed. Yet, for millions, it’s a daily reality of debilitating.

Pharmaceutical Technology
MARCH 25, 2025
Trump hinted that the US will impose a 25%+ tariff on pharmaceuticals after giving US companies time to build more manufacturing capacity.

Advertisement
Planning on running clinical trials in Japan? How can you reliably supply these studies? Discover Catalent’s clinical supply packaging facility in Shiga, Japan. Strategically located between Tokyo and Osaka, and one of largest in Japan, this 6,000 square meter facility offers comprehensive services including primary and secondary clinical packaging and labelling, comparator sourcing, cold chain storage, local and global distribution, local language support and white glove service to support stud
Let's personalize your content