Lilly’s tirzepatide cuts diabetes risk, study data show
Bio Pharma Dive
AUGUST 20, 2024
Treatment with the GLP-1 drug, which Lilly sells as Mounjaro and Zepbound, lowered the risk of Type 2 diabetes progression by 94% versus placebo.

Bio Pharma Dive
AUGUST 20, 2024
Treatment with the GLP-1 drug, which Lilly sells as Mounjaro and Zepbound, lowered the risk of Type 2 diabetes progression by 94% versus placebo.

Pharmaceutical Technology
AUGUST 20, 2024
Supplies of Emergent’s smallpox vaccine ACAM2000 will accompany Bavarian Nordic’s vaccine to African countries.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

AuroBlog - Aurous Healthcare Clinical Trials blog
AUGUST 20, 2024
Cerebrospinal fluid, or CSF, is a clear, colorless liquid that plays a crucial role in maintaining the health and function of your central nervous system. It cushions the brain and spinal cord, provides nutrients and removes waste products. Despite its importance, problems related to CSF often go unnoticed until something goes wrong.
Pharmaceutical Technology
AUGUST 20, 2024
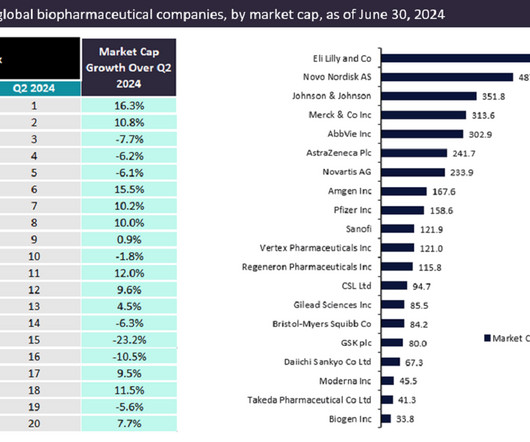
The top 20 biopharmaceutical companies demonstrated resilience during the second quarter (Q2) of 2024 as global markets and investor optimism improved with anticipation of a potential interest rate cut from the US Federal Reserve.

Speaker: Simran Kaur, Founder & CEO at Tattva Health Inc.
The healthcare landscape is being revolutionized by AI and cutting-edge digital technologies, reshaping how patients receive care and interact with providers. In this webinar led by Simran Kaur, we will explore how AI-driven solutions are enhancing patient communication, improving care quality, and empowering preventive and predictive medicine. You'll also learn how AI is streamlining healthcare processes, helping providers offer more efficient, personalized care and enabling faster, data-driven

Bio Pharma Dive
AUGUST 20, 2024
Approval of Rybrevant and Lazcluze was supported by results from a study that compared the regimen to AstraZeneca’s widely used drug Tagrisso.

AuroBlog - Aurous Healthcare Clinical Trials blog
AUGUST 20, 2024
The integration of biotechnology and nanotechnology into the Indian nutraceutical industry is setting a new standard for scientifically-backed health solutions, said Karthik Kondepudi, partner, Herbochem. The industry is on a trajectory of rapid growth and innovation, driven by technological advancements, changing consumer preferences, and a supportive regulatory framework.
Clinical Research Informer brings together the best content for clinical researchers from the widest variety of industry thought leaders.
Pharmaceutical Technology
AUGUST 20, 2024
As obesity rates rise and trust in GLP-1R products grows, sales are forecast to increase four-fold from 2023 to 2030.

Fierce Pharma
AUGUST 20, 2024
Regulators in both the U.S. and Europe have looked into the potential link between suicidal thoughts and Novo Nordisk’s blockbuster semaglutide franchise after reports sounded the alarm last year. | Researchers found that users of Novo's semaglutide who were logged in a WHO database were more likely to report suicidal ideation.

Pharmaceutical Technology
AUGUST 20, 2024
On July 2024, Colombia's President Gustavo Petro signed Law 2386 of 2024, which declared the pharmaceutical sector as strategic, at least in writing.

Fierce Pharma
AUGUST 20, 2024
As House lawmakers prepare for a September vote on the controversial BIOSECURE Act, the House Select Committee on the CCP is expanding the scope of its scrutiny on China's biopharma ecosystem. | As House lawmakers prepare for a September vote on the controversial BIOSECURE Act, the House Select Committee on the CCP is expanding the scope of its scrutiny on China's biopharma ecosystem.

Pharmaceutical Technology
AUGUST 20, 2024
J&J said the drug combination is now the first and only multitargeted, chemotherapy-free combination regimen proven to be superior to Tagrisso.

Rethinking Clinical Trials
AUGUST 20, 2024
The NIH Pragmatic Trials Collaboratory has launched a new interactive learning path that provides essential knowledge to research teams on how to choose the most appropriate study design for a pragmatic clinical trial. The learning path is a series of self-paced training modules that include expert videos, reference materials, and knowledge checkpoints.
Pharmaceutical Technology
AUGUST 20, 2024
Adcendo will acquire ex-China global rights for Multitude’s tissue factor-targeting ADC.

pharmaphorum
AUGUST 20, 2024
GSK gets FDA breakthrough status for its B7-H3 directed ADC, licensed from Hansah Pharma in a $1.

Advertisement
White paper that delves into the complex topic of Decentralized Clinical Trials and how to master them within the confines of FDA Regulations
Pharmaceutical Technology
AUGUST 20, 2024
ReAlta’s pegtarazimod is being developed to treat acute graft-versus-host disease (GvHD) for patients unresponsive to steroid therapies.

Fierce Pharma
AUGUST 20, 2024
Already wildly popular in type 2 diabetes and obesity, Eli Lilly’s tirzepatide can help prevent one of the very diseases it’s meant to treat, new data show. | Already wildly popular in type 2 diabetes and obesity, Eli Lilly’s tirzepatide can help prevent one of the very diseases it’s meant to treat, new data show.

Pharmaceutical Technology
AUGUST 20, 2024
Liquidia has received tentative approval from the US Food and Drug Administration (FDA) for YUTREPIA (treprostinil) inhalation powder.

Fierce Pharma
AUGUST 20, 2024
A new FDA approval has vaulted a Johnson & Johnson lung cancer drug combination into a key territory already dominated by AstraZeneca’s Tagrisso. | A new FDA approval has vaulted a Johnson & Johnson lung cancer drug combination into a key territory already dominated by AstraZeneca’s Tagrisso. But the real battle against the EGFR king has yet to start.

Pharmaceutical Technology
AUGUST 20, 2024
Accenture has made an investment in biotechnology company Earli for advancing early cancer detection technologies.

Fierce Pharma
AUGUST 20, 2024
For years, Teva has been defending itself against allegations that it used illegal Medicare co-pay schemes to fuel sales of its multiple sclerosis drug Copaxone. | The new lawsuit, filed in a Kansas court, comes as Teva defends against similar allegations made by the U.S. government.
Pharmaceutical Technology
AUGUST 20, 2024
The European Commission (EC) has granted conditional marketing authorisation for AbbVie’s TEPKINLY (epcoritamab).

Bio Pharma Dive
AUGUST 20, 2024
J&J has agreed to pay $600 million upfront for the private company as it works to expand its portfolio of cardiovascular devices.

Advertisement
This new white paper defines and details the impact of Decentralized Clinical Trials on the Pharmaceutical industry and how the impact can be measured along with steps companies can take to ensure adoption.

Pharmaceutical Technology
AUGUST 20, 2024
China’s National Medical Products Administration (NMPA) Center for Drug Evaluation (CDE) has approved Astellas’ PADCEV (enfortumab vedotin).
pharmaphorum
AUGUST 20, 2024
Learn about the exciting potential of CDK9 inhibitors in disrupting cancer cell growth and changing the treatment landscape for cancer patients. Stay updated on the latest research and developments in this promising field.

Pharma Times
AUGUST 20, 2024
The company’s Cell & Gene Therapy Centre can now offer a full range of GMP services

pharmaphorum
AUGUST 20, 2024
Avidity Bio raises $345m from its second big public offering of 2024, after reporting encouraging Duchenne muscular dystrophy data.

Advertisement
Planning on running clinical trials in Japan? How can you reliably supply these studies? Discover Catalent’s clinical supply packaging facility in Shiga, Japan. Strategically located between Tokyo and Osaka, and one of largest in Japan, this 6,000 square meter facility offers comprehensive services including primary and secondary clinical packaging and labelling, comparator sourcing, cold chain storage, local and global distribution, local language support and white glove service to support stud

Pharma Times
AUGUST 20, 2024
Osteoporosis currently affects 3.

pharmaphorum
AUGUST 20, 2024
Emergent BioSolutions has agreed to donate 50,000 doses of its mpox vaccine for use in the current outbreak, but an expert has warned supplies are unlikely to meet demand

Pharmaceutical Commerce
AUGUST 20, 2024
The latest news for pharma industry insiders.

pharmaphorum
AUGUST 20, 2024
Eli Lilly's latest clinical readout for dual GIP/GLP-1 agonist tirzepatide is a big one, with data suggesting the drug can reduce the risk of developing type 2 diabetes in overweight and obese adults with pre-diabetes.There's plenty of evidence to show that prediabetes – a higher-than-normal blood sugar level resulting from a level of insulin resistance that isn't high enough to be considered full-blown diabetes – can be reversed if patients commit to an intensive regimen of dietary restriction

Advertisement
Clinical trial data management is increasingly challenging as studies grow in complexity. Quickly accessing and analyzing study data is vital for assessing trial progress and patient safety. In this paper, we explore real-time data access and analysis for proactive study management. We investigate using adverse event (AE) data to monitor safety and discuss a clinical analytics platform that supports collaboration and data review workflows.
Let's personalize your content